1. INTRODUCTION
Sustainable alternative energy solutions need to be developed to prevent energy and environmental crises caused by increasing global energy consumption. Thermoelectric technologies, which can directly convert waste heat to electrical energy, have long attracted attention as a promising alternative energy solution. The conversion efficiency of thermoelectric devices is described by a dimensionless figure of merit (ZT = Žā╬▒2T╬║-1), where Žā: electrical conductivity, ╬▒: Seebeck coefficient, T: temperature in Kelvin, and ╬║: thermal conductivity). Therefore, to achieve a high ZT value, a high power factor (Žā╬▒2) and low thermal conductivity are required.
Recently, eco-friendly materials composed of nontoxic and earth-abundant elements have been extensively explored for thermoelectric devices. Among them, Cu-based ternary chalcogenides have received significant attention as promising materials for photovoltaic and thermoelectric applications [1-3]. Two of these, Cu3SbS4 (famatinite; space group I m) and Cu3SbSe4 (permingeatite; space group I m) are considered promising p-type thermoelectric materials in the intermediate-temperature range because of their narrow bandgap energy and low thermal conductivity [4,5]. Typically, two compounds with the same crystal structure can easily form a solid solution. This process is particularly suitable for thermoelectric materials when the guest atoms scatter phonons rather than electrons [6]. Cu3SbS4 and Cu3SbSe4 have a zinc-blende-type tetragonal structure, and they can form substitutional solid solutions because the differences in their atomic radii, and electronegativity between S2- and Se2-, are small. The substitution of Se for S causes lattice distortion due to different bonding interactions [7] and affects the effective mass of the valence bands [8]. Subsequent changes in the thermal and electrical properties and crystal lattice can be expected following the formation of solid solutions.
The melting method generally used to synthesize chalcogenides requires slow heating/cooling rates because of the high vapor pressure of the chalcogen elements and the long annealing time required for phase transformation and homogenization [6,7,9]. As a solution to the aforementioned problems, Cu3SbS4-Cu3SbSe4 solid solutions can be synthesized via mechanical alloying (MA) (high-energy ball milling) at room temperature. In our previous studies [10,11], Cu3SbS4 and Cu3SbSe4 powders were synthesized using MA and consolidated using hot pressing (HP), and the optimal conditions of MA (at 350 rpm for 12 h) and HP (at 623 K and 70 MPa for 2 h) were determined. In this study, solid solutions of Cu3SbS4-ySey (y = 0ŌĆō4) were prepared using MA and HP as a solid-state method, and their thermoelectric properties were investigated.
2. EXPERIMENTAL PROCEDURE
Solid solutions of Cu3SbS4-ySey (y = 0, 0.8, 1.6, 2.4, 3.2, and 4), Cu (purity 99.9%, < 45 ┬Ąm), Sb (purity 99.999%, < 75 ┬Ąm), S (purity 99.99%, < 75 ┬Ąm), and Se (purity 99.9%, < 10 ┬Ąm) were weighed according to the corresponding stoichiometric composition. The mixed powders were loaded into a hardened steel jar with steel balls in an Ar atmosphere. MA was conducted using a planetary ball mill to synthesize the solid solutions, and then HP was performed to consolidate the synthesized powders.
X-ray diffraction (XRD; D2-PHASER, Bruker) was conducted to identify the phases of the MA powders and HP compacts, and Rietveld refinement (TOPAS, Bruker) was performed to estimate their lattice constants. Thermogravimetry and differential scanning calorimetry (TG-DSC; TG/DSC1, Mettler Toledo) were employed to analyze the mass changes and phase transformations of the MA powders and HP compacts. Field-emission scanning electron microscopy (FESEM; JSM-7610F, JEOL,) was used to observe the microstructures. Energy-dispersive spectroscopy (EDS; X-Max50, Oxford) was used to analyze the compositions and elemental distributions with the following energy levels: Cu L-series (0.930 eV), Se L-series (1.379 eV), S K-series (2.307 eV), and Sb L-series (3.604 eV).
The van der Pauw method was used to examine the Hall coefficient, carrier concentration, and mobility. ZEM-3 (ADVANCE RIKO) equipment was employed to measure the Seebeck coefficient and electrical conductivity. A laser flash TC-9000H (ADVANCE RIKO) system was used to measure thermal diffusivity, and then the thermal conductivity was estimated from the theoretical density and specific heat. Finally, the thermoelectric power factor and figure of merit were evaluated.
3. RESULTS AND DISCUSSION
The XRD patterns of the MA powders and HP specimens of Cu3SbS4-ySey are shown in Fig 1. All the solid solutions were prepared without secondary phases via the MA-HP process. As the Se content increased, all of the diffraction peaks shifted to lower diffraction angles. This indicated that the lattice constants were increased by the formation of substitutional solid solutions of Cu3SbS4 (PDF# 00-035-0518, famatinite) and Cu3SbSe4 (PDF# 01-085-0003, permingeatite).
The estimated lattice constants of the Cu3SbS4-ySey solid solutions are shown in Fig 2. Cu3SbS4 had lattice constants of a = 0.539 nm and c = 1.074 nm, and Cu3SbSe4 had lattice constants of a = 0.565 nm and c = 1.124 nm. Sugaki et al. [12] reported lattice constants of a = 0.539 nm and c = 1.075 nm for Cu3SbS4, and Pfitzner [13] reported lattice constants of a = 0.566 nm and c = 1.128 nm for Cu3SbSe4. Because the ionic radius of Se (0.198 nm) is larger than that of S (0.184 nm) [14], the lattice constants of the solutions increased almost linearly with increasing Se content, according to VegardŌĆÖs law. This linear increase in lattice constants indicates that Se atoms were dissolved successfully into the S sites.
The results of the TG-DSC analyses of the hot-pressed Cu3SbS4-ySey solid solutions are shown in Fig 3. In previous studies [10,11], the melting points of Cu3SbS4 and Cu3SbSe4 were determined to be 823 K and 734 K, respectively, and no phase transformation or decomposition was observed at these temperatures. In this study, the melting points of Cu3SbS4-ySey decreased with increasing Se content (y): the melting points were 818 K for y = 0.8, 778 K for y = 1.6, 752 K for y = 2.4, and 743 K for y = 3.2. As shown in Fig 3(a), mass loss occurred at temperatures above the melting points, which was mainly due to the volatilization of the chalcogen elements. For Cu3SbS4, the large endothermic peak at 880 K corresponds to the melting point of skinnerite (Cu3SbS3) [10].
Figure 4 displays FESEM images of the polished (backscattered electron mode) and fractured surfaces of the Cu3SbS4-ySey. All the specimens contained only a few pores, and their relative densities were higher than 97.8%. The theoretical density of famatinite (Cu3SbS4) was reported to be 4.64 gcm-3 [15], and that of permingeatite (Cu3SbSe4) was reported to be 5.86 gcm-3 [16]. The theoretical densities of the Cu3SbS4-ySey solid solutions (0.8 Ōēż y Ōēż 3.2) were simply assumed and estimated using the rule of mixtures with Cu3SbS4 and Cu3SbSe4. The microstructures showed no significant differences despite the various Se contents. The actual compositions were similar to the nominal compositions (Table 1), and all the constituent elements were observed to be homogeneously distributed in the EDS line scans.
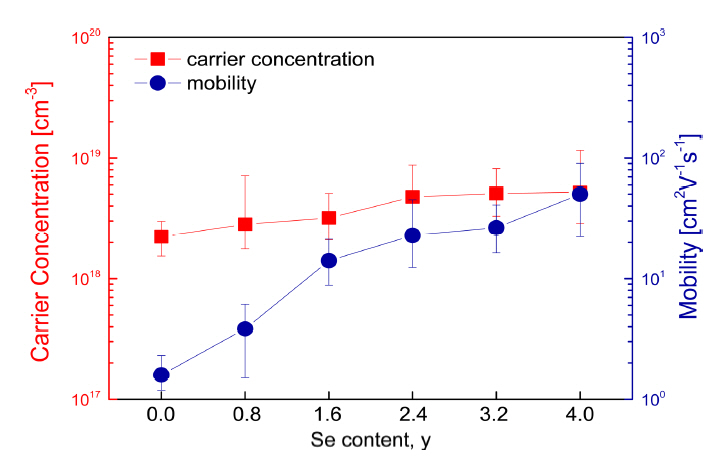
Figure 5 shows the electrical conductivity of Cu3SbS4-ySey. The positive temperature dependence of the electrical conductivity indicates nondegenerate semiconductor behavior. The temperature dependence of the electrical conductivity decreased with increasing Se content, but the electrical conductivity increased at a constant temperature. To elucidate the change in electrical conductivity, the carrier concentration and mobility were measured (Fig 6). Cu3SbS4 had a carrier concentration of 2.2├Ś1018 cm-3 and a mobility of 1.6 cm2 V-1s-1, whereas Cu3SbSe4 had a carrier concentration of 5.2├Ś1018 cm-3 and a mobility of 49.9 cm2 V-1s-1. As the Se content increased, the carrier concentration and mobility increased, and thus the electrical conductivity increased.
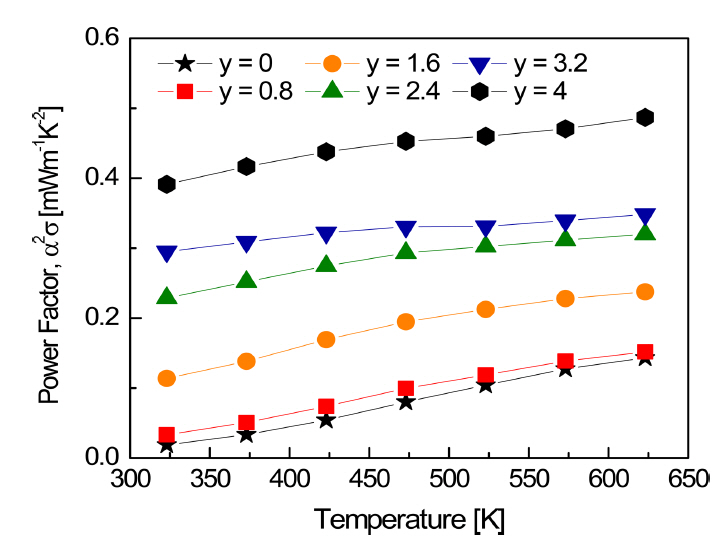
On the other hand, the Seebeck coefficient decreased with increasing Se content, as shown in Fig 7. According to the Pisarenko relation [17], the Seebeck coefficient decreased with increasing Se content, owing to the increase in the carrier concentration. The intrinsic carrier concentration (ni) is closely related to the bandgap energy, according to the following equation [18]: ni = CT3/2exp[-Eg/2kBT] (C: constant, Eg: bandgap, and kB: Boltzmann constant). Cho et al. [19] reported that as the Te content increased in SnSe-SnTe solid solutions, the Seebeck coefficient decreased due to the increase in carrier concentration caused by the reduction in bandgap. The bandgap of Cu3SbS4 ranges from 0.47 eV to 0.9 eV [20,21], and that of Cu3SbSe4 has been reported to be ~0.29 eV [4]. Therefore, the bandgap was decreased by the formation of a Cu3SbS4-Cu3SbSe4 solid solution. As the intrinsic carrier concentration increased, it in turn changed the electrical conductivity and Seebeck coefficient. As the Se content increased, the power factor values of the solid solutions lay between those of Cu3SbS4 and Cu3SbSe4 (Fig 8). This suggests that optimization of the carrier concentration through doping is required to improve the power factor.
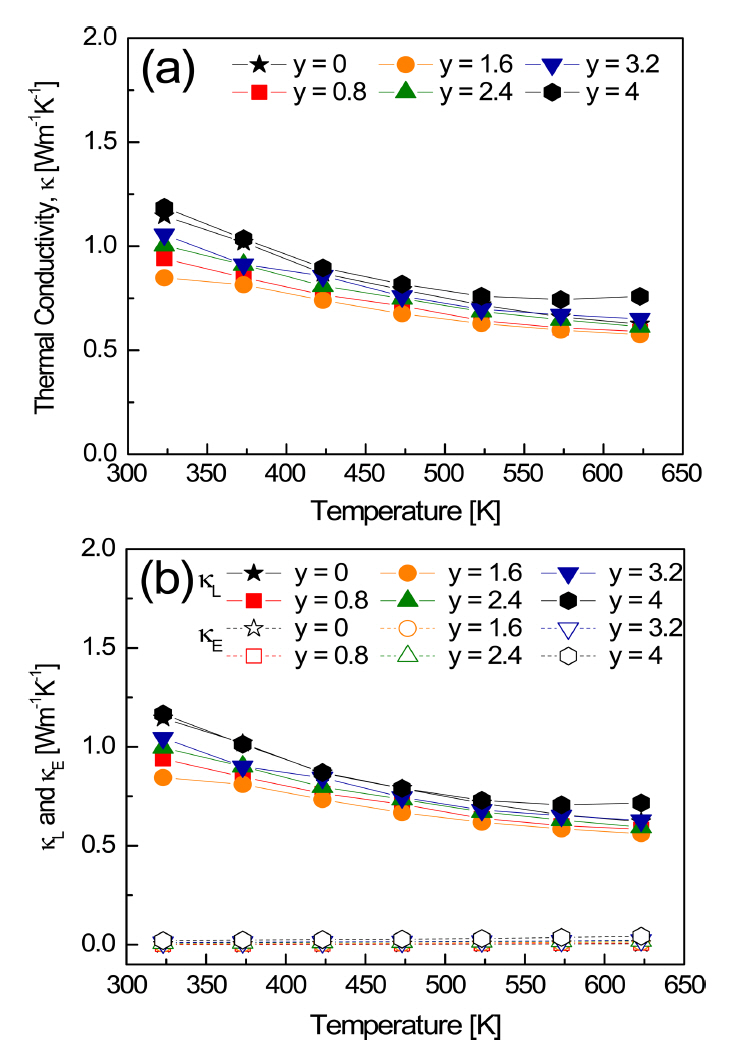
Figure 9 shows the thermal conductivity of Cu3SbS4-ySey. The thermal conductivity is the result of contributions from the charge carrier transport (╬║E, electronic thermal conductivity) and lattice vibrations (╬║L, lattice thermal conductivity). The WiedemannŌĆōFranz law (╬║E = LŽāT) was applied to separate the electronic thermal conductivity; the temperature-dependent Lorenz number (L) was obtained using the equation [22], L = 1.5 + exp[ ]. In this study, the ranges of the Lorenz number were (1.51ŌĆō1.57) ├Ś 10-8 V2K-2 at 323 K and (1.51ŌĆō1.56) ├Ś 10-8 V2K-2 at 623 K. The Lorenz number increased slightly with increasing Se content. The estimated Lorenz numbers at 323 K are summarized in Table 1. The Lorenz number has a theoretical range of (1.45ŌĆō2.44) ├Ś 10-8V2K-2 [23], and the lower values indicate nondegenerate semiconductor behavior (the higher values indicate degenerate semiconductor or metallic behavior). This indicates that Cu3SbS4-ySey is a nondegenerate semiconductor.
The thermal conductivities of the solid solutions were lower than that of Cu3Sb(S/Se)4, and this was attributed to reduced lattice thermal conductivity resulting from lattice distortion. There was little contribution from the electronic thermal conductivity, because of the poor electrical conductivity. In addition, the thermal conductivity was less than 0.8 Wm-1K-1 at 623 K. These values were much lower than the values of 1.8ŌĆō2.2 Wm-1K-1 reported for the Cu3SbS4-ySey (y = 0.8, 1.2) solid solutions prepared via the melting process [24]. This can be attributed to the enhanced phonon scattering, following the introduction of numerous grain boundaries during MA.
Figure 10 shows the variation in thermoelectric properties at 323 K with Se content for Cu3SbS4-ySey. As mentioned previously, as the Se content (i.e., Cu3SbSe4 content) increased, the intrinsic carrier concentration increased, due to the reduction in bandgap energy. As a result, the electrical conductivity increased, while the Seebeck coefficient decreased, resulting in an increase in the power factor. The electronic thermal conductivity increased with increasing Se content. However, the specimen with y = 1.6 exhibited the lowest lattice thermal conductivity, and thus, the lowest thermal conductivity. This was due to the alloying effect (phonon scattering) caused by the difference in atomic mass between S (32.07 gmol-1) and Se (79.86 gmol-1) [25,26]. The lowest lattice thermal conductivity was 0.84 Wm-1K-1 for Cu3SbS2.4Se0.16, which was mainly attributed to the enhanced phonon scattering caused by strain field fluctuations due to the differences in atomic radius and mass.
Figure 11 shows the ZT values of Cu3SbS4-ySey. As the temperature and Se content increased, the ZT values increased. All the solid solutions (0.8 Ōēż y Ōēż 3.2) exhibited ZT values between those of the end compounds (y = 0 or y = 4) without further improvement, despite the reduction in lattice thermal conductivity. However, the formation of solid solutions did effectively decrease thermal conductivity, and controlled the electrical properties. The thermoelectric performance was expected to be improved by maximizing the power factor, by optimizing the carrier concentration.
4. CONCLUSIONS
Solid solutions of Cu3SbS4-ySey (y = 0ŌĆō4) were prepared using MA and HP. The lattice constants increased linearly with increasing Se content, indicating the successful formation of Cu3SbS4-ySey solid solutions. The signs and values of the Hall and Seebeck coefficients confirmed p-type conduction and nondegenerate semiconductor characteristics. As the Se content increased, the carrier concentration and mobility increased; as a result, electrical conductivity increased, while the Seebeck coefficient decreased. Consequently, the power factor increased. The thermal conductivity was lowered by the formation of the solid solutions, and the contribution (phonon scattering due to the lattice distortion) of the lattice thermal conductivity was dominant. These results confirmed the effect of the solid solution on thermal conductivity, and doping will be required to improve the thermoelectric performance of Cu3SbS4-ySey solid solutions in further studies.