1. Introduction
ZnS, one of the II-VI semiconductor compounds with a polymorph crystal structure, is a material with a wide bandgap energy (3.5 eV~3.7 eV for the zinc-blende structure and 3.7 eV~3.9 eV for the wurtzite structure) and a large exciton binding energy of 40 meV. Because it has excellent optical and semiconductor properties, it can be used in a range of fields, for electroluminescence, light-emitting diodes, gas sensors, solar cells, bio devices, and ultraviolet light-based photosensors and photodetectors [1-9]. In addition, the photocatalytic properties of ZnS have attracted particular attention, given its high efficiency and chemical stability, properties which make it suitable for treating non-degradable and toxic pollutants present in wastewater [10-12].
Recently, various approaches to synthesize ZnS have been attempted, including solid-state reaction [13], the precipitation method [14], the sol-gel method [6], chemical bath deposition [10], the microwave method [15], the vaporliquid-solid (VLS) synthesis method [16], and the hydrothermal method [17,18]. The above methods require complex equipment and processes to manufacture the powder; in addition, a significant amount of energy is required to obtain crystalline powder.
As an alternative, a method similar to the hydrothermal method, the glycothermal method, can be used for synthesizing anhydrous crystalline materials in a nonaqueous solvent, using various glycols (1,4-butanediol, diethylene glycol, ethylene glycol, etc.), and dihydric alcohol, rather than water. The method has been employed in attempts to produce various powders [19,20]. Compared to the hydrothermal method, the glycothermal reaction can be performed at both lower temperature and pressure. The shape and size of the powder can be easily controlled without growth directing agents in a short reaction time. Inorganic mineralizers (KOH, NaOH, etc.), which can cause contamination, are not required. The glycothermal method does directly cause the formation of anhydrous crystalline materials in some cases, because glycols as a solvent can complex ions to promote solubility. Therefore, the role in terms of the properties of various glycols as a solvent is very important. However, the reaction mechanisms in nonaqueous solution are complex, and currently there is very limited information regarding the reaction thermodynamics, kinetics, and underlying crystallization mechanisms.
In this study, ZnS crystalline particle was produced using the glycothermal method with ethylene glycol as a reaction solvent. A simple process of direct glycothermal treatment in a solution in which a starting material was dissolved was used for synthesis at each reaction temperature. The properties of the synthesized particle were evaluated. ZnS has excellent optical properties due to the rapid generation of electron-hole pairs by photo-excitation and the high negative reduction potential of the excitation electrons, so it can be used as a photocatalytic material. To confirm the photodecomposition effect of organic pollutants, its photocatalytic properties were evaluated using an ultraviolet light source.
2. Experimental Procedure
In this study, zinc acetate dihydrate (Junsei, 99%) and thiourea (NH2CSNH2, DC Chemical, 98%) were used as the starting materials with Zn and S to synthesize ZnS particles using the glycothermal method. The reaction solvent was ethylene glycol (Duksan, 99%). After sufficiently dissolving the zinc acetate dihydrate and thiourea in 70 ml of ethylene glycol at 0.02 mol each, the dissolved solution was put into an autoclave apparatus with a 100 ml Teflon container and subjected to the glycothermal treatment. Particles were synthesized at each reaction temperature, with a heating jacket used for the glycothermal reaction. The synthesized precipitates were centrifuged three times for 15 minutes at 8000 rpm using ethanol to separate and wash the reaction solvent. After drying for 12 hours in a dryer at 100 °C, the final ZnS particle was obtained.
The crystal structure of the prepared ZnS particles were checked using an X-ray diffractometer (XRD, SmartLab, Rigaku, Japan) (CuKα1, 45 kV/200 mA, 4°/min, 2θ = 10-80°). The particle shape and its components were observed with a field emission scanning electron microscope (FE-SEM, S-4800, Hitachi, Japan) using energy dispersive spectroscopy (EDS), and a transmission electron microscope (TEM, JEM-2100F, Jeol, Japan) was used to observe the dispersibility and crystal phase of the synthesized particle. To check the composition and chemical bonding state on the particle’s surface, an X-ray photoelectron spectroscope (XPS, MultiLab, ESCA 2000) with an Al-Kα monochromator as an X-ray source and a concentric hemispherical analyzer was used. To measure the specific surface area of the particle, it was analyzed in a nitrogen atmosphere using BET (ASAP 2020, Micromeritics, USA). In addition, the optical properties of the ZnS particle obtained at each reaction temperature were analyzed and evaluated at room temperature using an ultraviolet-visible spectrophotometer (V-550, JASCO, Japan).
To investigate the photocatalytic properties of the ZnS particles obtained at each reaction temperature, a photodecomposition experiment was performed on an organic dye in a 10 ppm methyl orange (MO) aqueous solution. 100 ml of MO aqueous solution with 0.5 g of ZnS particle was irradiated while stirring in a dark room using a 30 W ultraviolet BLB lamp with a wavelength of 365 nm as an excitation source. In addition, to prevent the MO aqueous solution being evaporated by the heat generated from the UV lamp, it was kept at room temperature using a cooling reactor. The photodecomposition reaction of the MO organic dye was carried out for 30, 60, 90, 120, 150 minutes, 3 hours, and 4 hours. After separating the ZnS particles using a centrifuge to ensure they were not mixed, the aqueous solution was collected and put into a quartz cell following the photodecomposition reaction for each duration. Then, UV spectroscopy (300 nm~650 nm) was performed to evaluate the photodecomposition characteristics of the MO organic dyes.
3. Results and Discussion
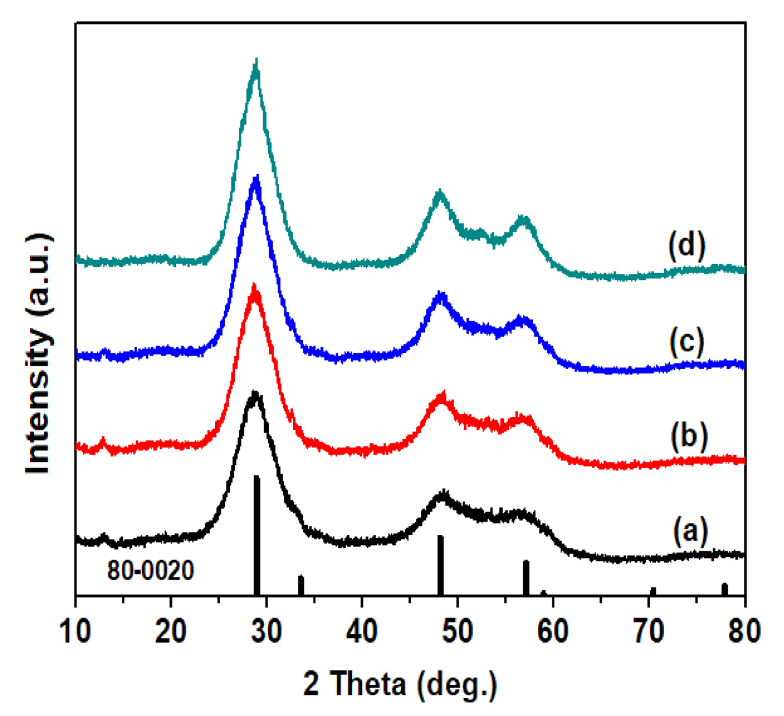
The ZnS particles obtained at reaction temperatures between 125 °C and 200 °C were subjected to an XRD analyses of their crystal structure, and the results are shown in Fig 1. ZnS was not synthesized at a reaction temperature of 100°C or less, and crystalline pure ZnS was synthesized at a reaction temperature of 125 °C or higher, a relatively low temperature. The main peaks of the synthesized ZnS XRD pattern were (111), (220), and (311), which correspond to 28.7 °, 48.1 °, and 57.0 °, and the lattice constant of ZnS calculated from the (111) diffraction peak showed that ZnS particles with a zinc-blende crystal structure of 5.38 Å was synthesized (JCPDS No. 80-0020). As the reaction temperature increased, the intensity of the XRD peak of the ZnS particles rose slightly, indicating that they had excellent crystallization. In addition, the produced ZnS particles had a wide full width at half maximum (FWHM) of the XRD peak regardless of the reaction temperature, and the average particle size calculated using Scherrer’s equation was approximately 4 nm [21].
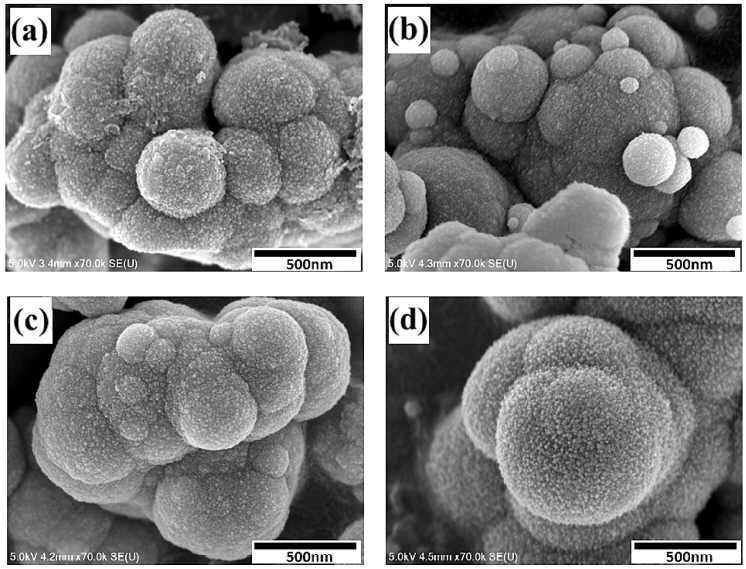
Fig 2 shows an FE-SEM image of the ZnS particles produced by glycothermal treatment for 1 hour at each reaction temperature. In general, the reaction temperature affects the growth and movement speed of the initial ZnS particles, and the particle diameter distribution [22]. When the reaction temperature of the precursor solution increases, thiourea is decomposed and it slowly and uniformly generates S2- ions, after which Zn2+ ions react with S2- ions to form ZnS nanocrystals. The ZnS particles produced at all reaction temperatures are formed by a continuous supply of uniform ZnS nanocrystals of primary particles, due to glycothermal reaction. These nanocrystals move to self-aggregate with each other and then form regular spherical secondary particles of sub-micron size. At a relatively low reaction temperature, spherical secondary particles with nonuniform sizes from 0.1 μm to 0.5 μm reacted with each other to form agglomerated ZnS particles. The formation and movement speed of the primary nanocrystals increased as the reaction temperature increased to 200 °C, and it was found that the size of the spherical secondary particles gradually grew to about 0.7 μm without changing shape, due to their self-aggregation with each other. In addition, it was found that the size of the initial ZnS nanocrystals on the surface of the spherical self-aggregated ZnS particles did not change, even when the reaction temperature was increased. It was further found that the porosity of the ZnS particles increased slightly. It is thought that the spherical self-aggregated ZnS particles formed through the self-aggregation process, followed by the Oswald aging process, because no additives such as surfactants or capping agents were used in this study [23].
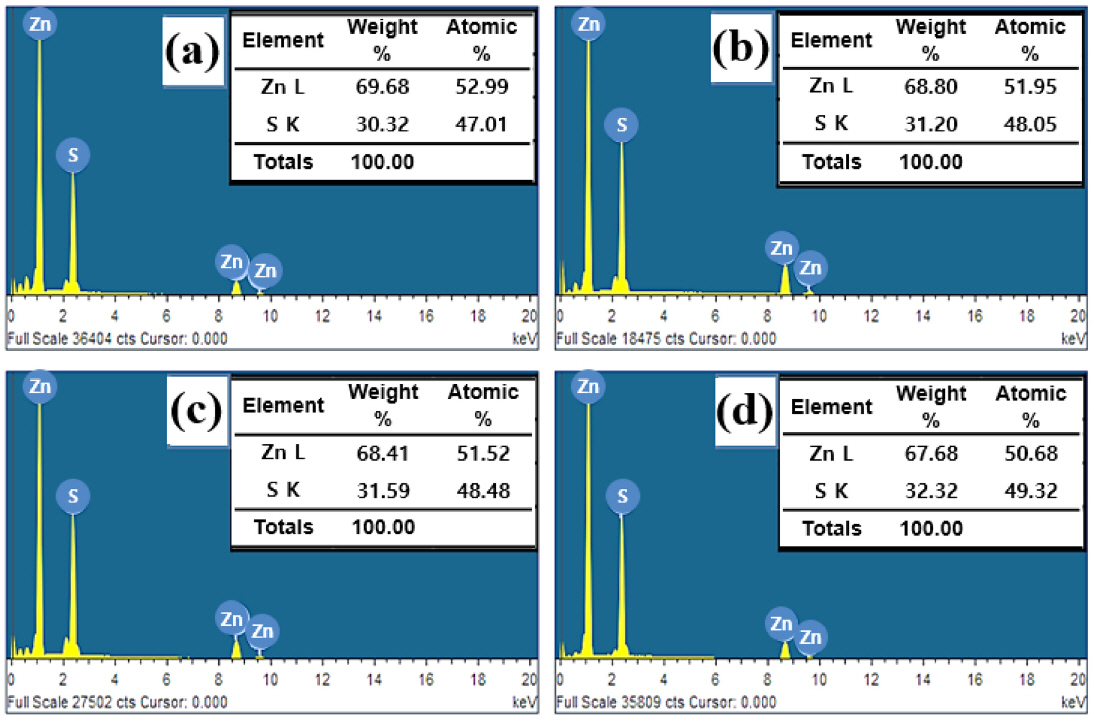
Fig 3 shows the EDS analysis results for the ZnS particles produced at each glycothermal reaction temperature. The EDS spectra of all the particles consisted of only Zn and S elements. At 125 °C, where the reaction temperature was relatively low, a ZnS particle lacking S was produced, as the atomic ratio of Zn to S was approximately 1:0.89. As the reaction temperature increased, the content of S increased, and a compound was found where the atomic ratio of Zn to S was close to a stoichiometric ratio of approximately 1:1.
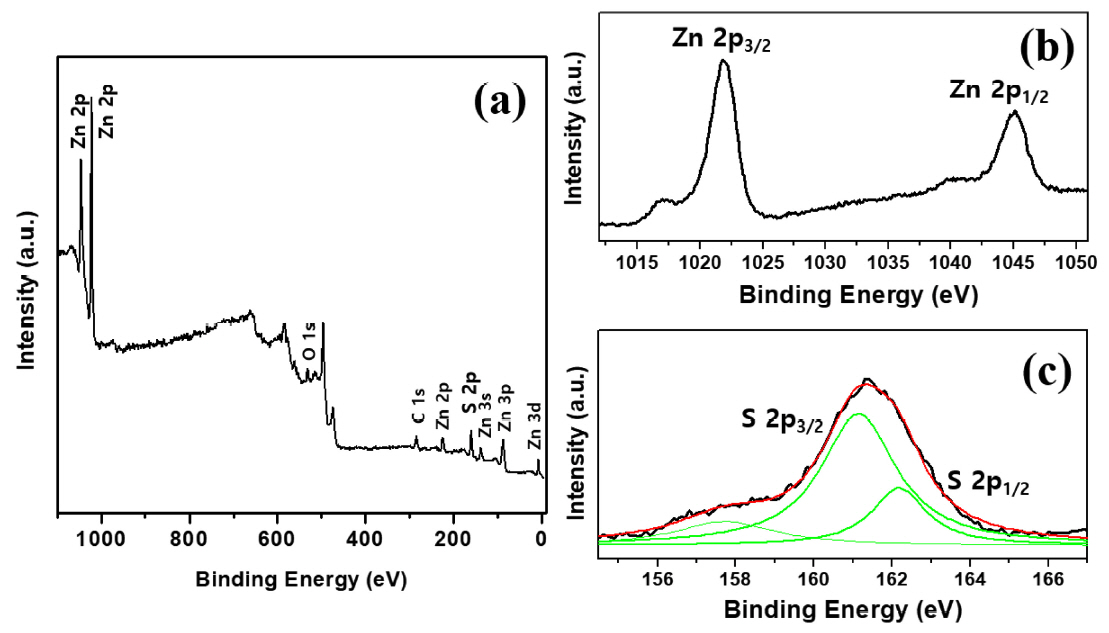
Fig 4 shows typical XPS results, analyzing the chemical bonding state of the constituent elements of self-aggregated ZnS particles produced through the glycothermal method at 200 °C for 1 hour. The spectra of Zn and S were observed. Internally, the spectral position of binding energy was used as a reference for calibration, based on the C 1s of 284.5 eV of the surface adventitious carbon. The weak spectra of C and O are considered to be from contamination when the particle surface was exposed to air. The peak of O 1s at 531.5 eV may come from adsorbed H2O or O2 on the surface of the particles [24], and is evidently not consistent with the O 1s signal of ZnO (530.5 eV) [25]. Furthermore, no particularly noticeable oxidized S species, such as -SO2, were detected [26]. Accordingly, the ZnS particle produced was a high purity compound without impurities. As shown in Fig 4(b), a ZnS particle having a zinc-blende structure was observed at 1021.9 eV and 1045.0 eV, corresponding to the binding energies of Zn 2p3/2 and 2p1/2, respectively. The asymmetric S 2p peak in Fig 4(c) was deconvoluted into two peaks corresponding to S 2p3/2 and S 2p1/2 and were located at 161.1 eV and 162.2 eV, respectively. The binding energy of 161.1 eV originated from S2− in the ZnS. The other sub-peak of approximately 162.2 eV formed due to S-S species [26,27].
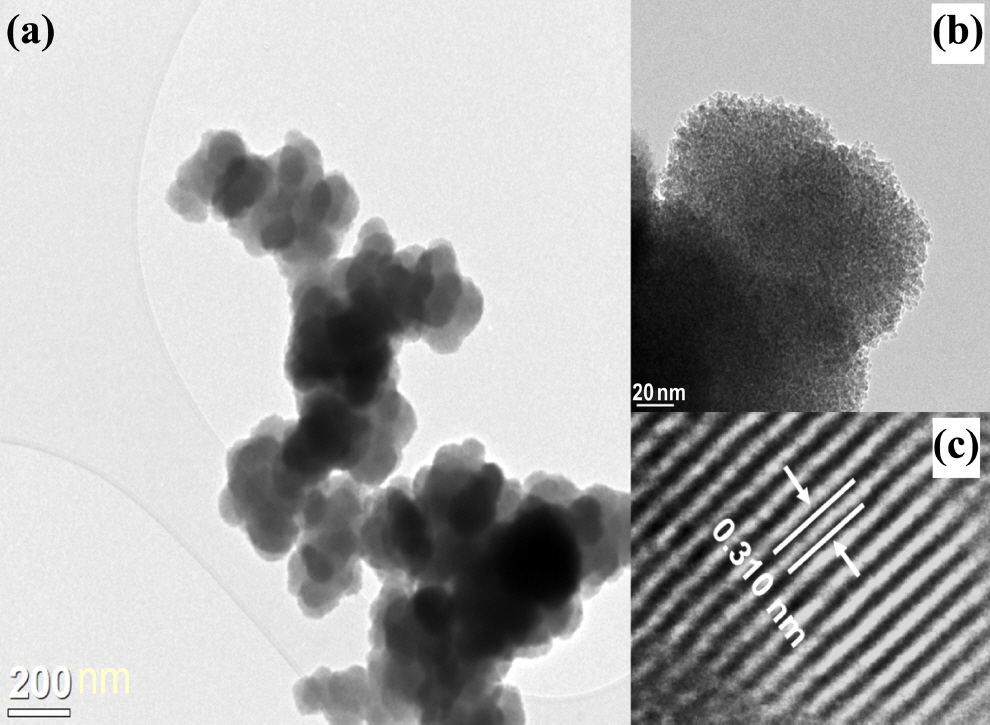
Fig 5 is a TEM image of a ZnS particle synthesized through the glycothermal method at 200 °C for 1 hour. It was found that the ZnS nanocrystals of the primary particles aggregated with each other to form secondary particles with an average particle size of 0.4 μm to 0.7 μm, with a nearly spherical shape. In high-resolution TEM images, the ZnS aggregates were found to be composed of uniform nanocrystals ranging from 4 nm to 5 nm in size. Their size was similar to the average particle size calculated using Scherrer’s equation. In addition, a lattice fringe with an interplanar spacing of 0.310 nm was determined to be the (111) fringe of a single crystal ZnS nanocrystal, and it was close to the zinc-blende lattice constant of ZnS.
Typical N2 gas adsorption-desorption isotherms for the ZnS particle synthesized at 200 °C for 1 hour are shown in Fig 6. It was found that the self-aggregated ZnS particle had a BET surface area of 156.1 m2/g and an average pore size and total pore volume of 3.4 nm and 0.087 cm3/g, respectively. The stated specific surface area was a high value compared to the mesoporous microsphere ZnS reported in the literature [26]. Although the self-assembly of ZnS microspheres has been reported, the macroporous ZnS corresponded to a type IV curve of the general IUPAC (International Union of Pure and Applied Chemistry) classification, and formed a mesopore structure between the particles on the microsphere surface. On the other hand, the adsorption-desorption isotherm obtained in Fig 6 is a curve close to type I classification. This is considered to indicate a microporous material with a broader pore size distribution, with wide micropores and narrow mesopores [28].
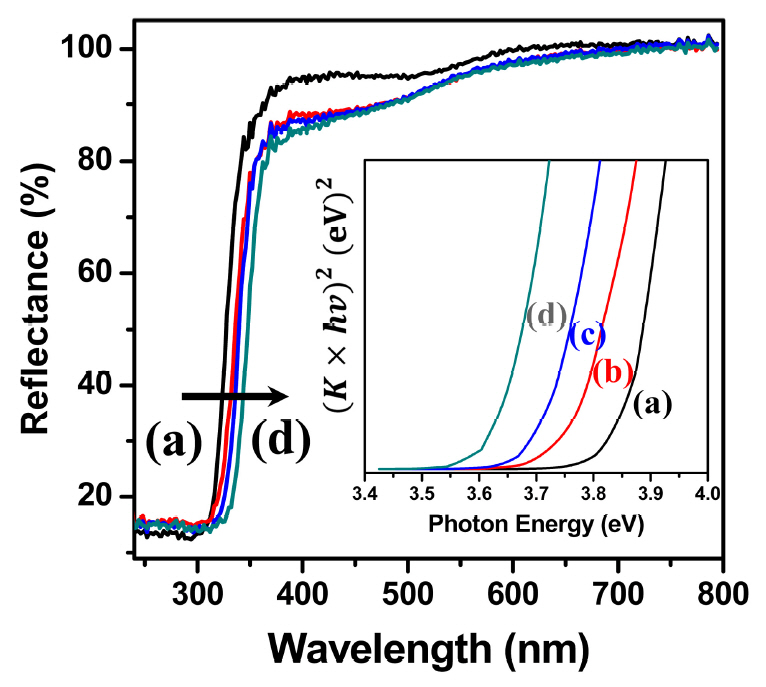
Fig 7 shows the results obtained with an ultraviolet-visible spectrophotometer and diffused reflectance spectra (DRS) of the self-aggregated ZnS particles synthesized by the glycothermal method at each reaction temperature. Observing the reflectance spectra of the particles produced at all reaction temperatures, it was found that strong absorption occurred in the range of about 300 nm to 370 nm, which suggests that they are potentially efficient photocatalysts for UV light-driven applications. The band gap energy was calculated using the following equation.
where, K is the Kubelka-Munk coefficient, R is the diffused reflectance (%), and hv is the photon energy. K is obtained from R using the above relational expression. It is converted into [K·hν]2 vs. photon energy as shown in the inset of Fig 7. The band gap energy of ZnS was calculated by the extrapolation of [K·hν]2 = 0. As the glycothermal reaction temperature increased, the absorption edge of ZnS was observed to move toward the longer wavelength, and the calculated band gap energy decreased to 3.85 eV, 3.76 eV, 3.70 eV, and 3.62 eV. The red-shift phenomenon of the energy band gap is reportedly due to an increase in particle size, and the internal stress in a crystal structure of small size [11,13]. It is obvious that the bandgap variation could be attributed to changes in the size of the spherical secondary particles with the increased reaction temperature, and the internal stress in the small primary self-aggregated ZnS particles. In addition, it can likely be ascribed to the formation of surface defects and sulfur vacancies in the particles, as shown in the EDS results [29].
Fig 8 shows the results of the UV spectroscopic analysis of the photocatalytic decomposition experiments using methyl orange (MO, λmax= 464 nm), an organic dye. To evaluate the decomposition characteristics of MO, the absorption spectral changes of the ZnS particle produced at 175 °C were measured. They showed a distinct decomposition phenomenon in relation to the UV irradiation time, as shown in Fig 8(a). As the UV irradiation time increased, the intensity of the MO absorption peak gradually decreased and about 90% of the MO was decomposed after 3 hours of irradiation. This indicates that the MO concentration decreased due to the destruction of the color development structure of the dye by the photocatalytic effect of the ZnS. To confirm the effect on the decomposition of MO, the change in MO photodecomposition was obtained using the following equation. UV irradiation was performed for different amounts of time, and the changes are shown in Fig 8(b) [11,30].
where, C0 is the initial MO concentration, Ct is the MO concentration after UV irradiation for a certain period of time, k is the reaction rate constant, and t is the UV irradiation time (h). A linear relationship plot of the pseudofirst-order reaction between ln (Ct/C0) and UV irradiation time was observed for all of the self-aggregated ZnS particles. Their rate constants (k), correlation coefficients (R2), and the kinetics equations can be obtained from the above equation, as shown in Table 1.
In the ZnS particles produced at 175 °C or higher, the reaction rate constant showed a steep slope, so that the dye MO rapidly decomposed within 60 minutes of irradiation, resulting in 60% of ZnS at 175 °C and 90% of ZnS at 200 °C. From Table 1, it can be concluded that the pseudo-first-order rate constant was 3 times faster when the reaction temperature was increased from 175 °C to 200 °C. In addition, the ZnS prepared at 200 °C showed excellent photocatalytic decomposition activities, with almost 100% decomposition in 90 minutes of irradiation. The self-aggregated ZnS particles prepared at 200 °C had a faster rate of reaction, corresponding to the maximum of k values, indicating outstanding photocatalytic activity. However, only about 10% decomposition of MO dye molecules was achieved by the ZnS particle prepared at 150 °C or less, even after 4 hours of irradiation.
In ZnS, a semiconductor compound, electron-hole pair carriers are generated by electrons excited in the conduction band and holes in the valence band due to the photoexcitation phenomenon. Super oxide anion radicals are generated by electrons photoexcited in the conduction band. The holes in the photo-induced valence band react with water and hydroxide anions to form hydroxyl radical species that are assumed to play a role in dye decomposition. The photodecomposition behavior of MO with the ZnS photocatalyst can be expressed using the following equation.
(3)
Recent studies have reported that the efficiency of photodecomposition can be improved by controlling the particle size, specific surface area, and band gap of the photocatalyst materials [26,30-32]. In this study, although the size of the secondary particles of the produced ZnS particle increased when the glycothermal reaction temperature increased, excellent photocatalytic activities were obtained because of its crystallization, and also because the particle is made of self-aggregated and uniform small primary nanocrystals. This leads to a large reaction-specific surface area. In addition, when photo-excited by a UV light source of 365 nm, the ZnS particles produced at 150 °C or less had a wider band gap. Therefore, sufficient photo-excitation did not occur. However, the ZnS particles produced at 175 °C or higher showed an improved photodecomposition efficiency by generating electron-hole pair carriers with sufficient photoexcitation in a narrow band gap.
4. Conclusions
In this study, self-aggregated ZnS crystalline particles were successfully produced at various reaction temperatures using the glycothermal method, a simple and environmentally friendly particle manufacturing method. The physical properties of the particles produced and the photodecomposition characteristics of the photocatalytic material were investigated using methyl orange (MO) dye. The ZnS particle crystallized at a relatively low reaction temperature of 125 °C was found to have a zinc-blende crystal structure. The ZnS particle sizes could be controlled by the glycothermal reaction temperature. An agglomerated secondary particle with a size of about 0.5 μm to 0.7 μm and a spherical shape were formed from uniform primary nanocrystals of about 4 nm as the reaction temperature increased. The self-aggregated ZnS particles were produced by the glycothermal method at 200 °C for 1 hour without using any additives such as surfactants or capping agent. The result was a high purity ZnS compound without impurities, with a theoretically stoichiometric ratio. The photodecomposition properties of the self-aggregated ZnS particle were measured with MO, and it was found that as the irradiation time under a UV light source (365 nm) increased, the MO concentration decreased as the MO was decomposed. The rate of photodecomposition was high for the self-aggregated ZnS particles produced at 175 °C or higher. In particular, the self-aggregated ZnS particle produced at 200 °C had a large specific surface area and a narrow energy band gap of about 3.62 eV in a UV light source, so that the electron-hole pair carriers generated by photoexcitation improved the photodecomposition efficiency of the organic dye. For each different glycothermal reaction temperature, a self-aggregated ZnS particle with excellent crystallization was obtained, and it was found that it significantly affected the photocatalytic decomposition performance.