1. INTRODUCTION
The acousto-optic (AO) effect has been used to selectively detect specific wavelengths from an incident light source. The essential components of an acousto-optic modulator (AOM), a single crystal and piezoelectric transducer, enable an acousto-optic tunable filter (AOTF) module to effectively modulate the light source. By adjusting the vibration frequency of the piezoelectric transducer, the AOM can detect a specific wavelength from the incident light, using diffraction. AOTF modules can be used in a wide range of applications including medical diagnostics, mineral exploration, air quality monitoring, and the detection of harmful biological agents [1-7]. However, to classify toxic aerosols which are detected in the infrared region of 8–12 µm, high quality AOM materials are needed. The grade of the grown AO single crystal determines the performance of the acousto-optic device. Diverse AO materials including Te, thallium arsenic selenide (Tl3AsSe3 or TAS), chalcogenide glasses (As2S3 and As2Se2) and mercurous halides (Hg2Cl2 and Hg2Br2) have been insensitively investigated for this purpose [8-14]. Although Te has the highest performance index for an AOM material, it is prone to being easily degraded by heat. Meanwhile, TAS and chalcogenide glasses involve relatively high facility construction costs for crystal growth [15]. Non-toxic chalcogenide glasses such as Ga2S3 also have a relatively low figure of merit [16]. On the other hand, mercurous bromide (Hg2Br2) has several merits including a broad optical transmission band (0.4–30 µm), a wide birefringence, a high refractive index, and a relatively high figure of merit, which makes it a promising AOM material. Although various studies about growth involving Hg2Br2 have been carried out, there are few reports addressing the full synthesis process, from the purification of Hg2Br2 raw powder to optimization of crystal growth. Since material purity and the crystal growth conditions strongly affect the formation of atomic and bulk defects in PVT-based Hg2Br2 crystal, the entire process should be carefully and systematically investigated.
In this study, we purified Hg2Br2 powder using the preclean and PVT processes. The main components of the Hg2Br2 powder were characterized by X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) analysis. In addition, the purification process was analyzed and verified by inductively coupled plasma (ICP). The crystallinity and lattice bonding of the grown crystal were also confirmed through XRD analysis and Raman vibration peaks. Better understanding of the purification and crystal growth process will contribute to the development of high quality crystal and high performance AOTF devices.
2. EXPERIMENTAL DETAILS
Purification of Hg2Br2 powders. The Hg2Br2 powders were purified through the PVT process prior to crystal growth. To remove the impurities in the quartz ampoule, it was sequentially cleaned in a series of wet chemicals including high-purity acetone, isopropyl alcohol, and ultrapure water. The cleaned ampoule was dried at 150 °C for 1 hour at 10-3 torr to remove any residual moisture existing inside the ampoule. After that, four-nine grade (99.99%) Hg2Br2 powder was put into the ampoule and again dried for 1 hour at 100 °C at 10-5 torr to completely evaporate the unintentional residual moisture. Lastly, the Hg2Br2 raw powder was purified at 300 °C for 10 hours under the high vacuum of 10-5 torr. To prevent the nucleation of Hg atoms and related secondary phases, the chamber was very slowly cooled at a rate of 1 °C/min. A highly purified powder with five-nine grade (99.999%) was obtained.
Hg2Br2 crystal growth process. After finalizing the purification process, the Hg2Br2 powder was charged into a quartz ampoule and a high vacuum of 10-6 torr was made. To sublimate the Hg2Br2 raw powder and then transfer the sublimated sources to the growth zone, a temperature gradient profile was formed between lower and upper heaters, with temperatures of 335 and 360℃ for each region, respectively. The sublimated powder was condensed in the source zone at the top of the ampoule and then Hg2Br2 crystal seed was formed in the growth zone at the bottom of the ampoule. Hg2Br2 crystals grew from the seed at a rate of 0.2 to 5 mm/day under the optimized temperature gradient of the ampoule. When the final crystal reached a diameter of 35 mm and was 70–80 mm in length, the crystal experienced a slow cooling down to prevent cracks from shrinkage.
Material characterization of Hg2Br2. The Hg2Br2 powder and crystal were characterized by XRD (JP/SmartLab, Rigaku, Tokyo, Japan) and XPS (PHI Quantera-II by Ulvac-PHI, Osaka, Japan). Impurities in the Hg2Br2 powder before and after purification were analyzed using ICP-OES (Optima 7300DV by Perkin Elmer, MA, USA) and ICP-MS (7900 ICP-MS by Agilent Technologies, CA, USA). Raman spectroscopy analysis was conducted to observe the unique atomic vibration modes of the grown Hg2Br2 crystal using a 532-nm green laser as the excitation source.
3. RESULTS AND DISCUSSION
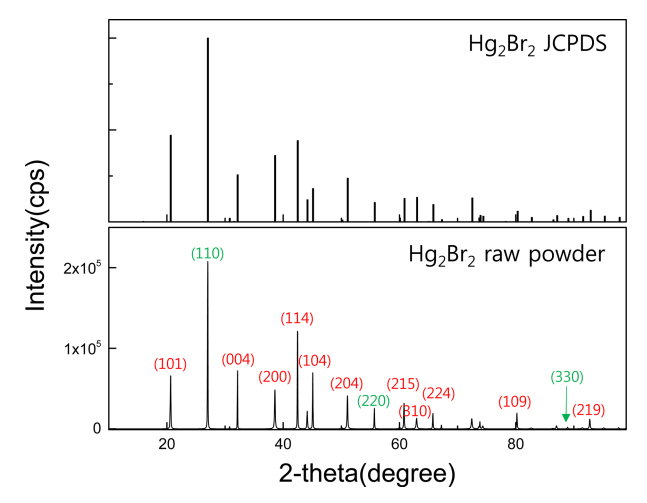
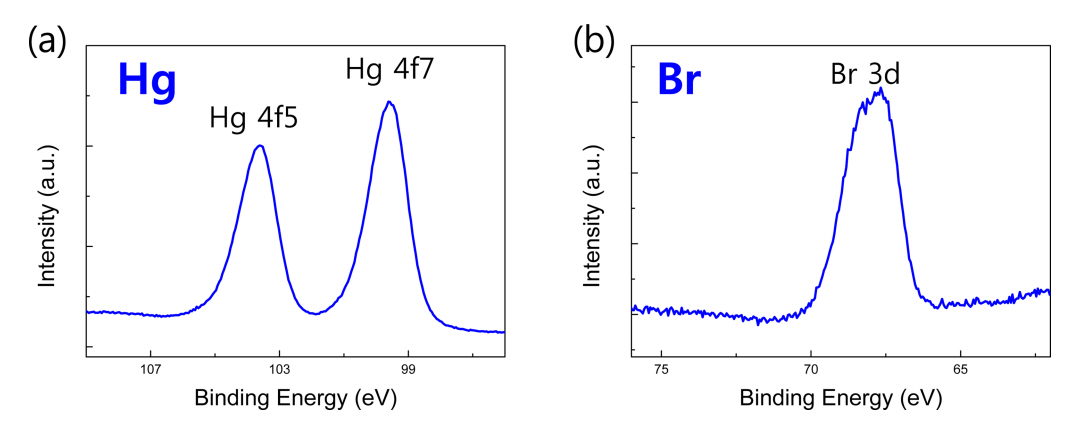
The diffraction pattern of the raw Hg2Br2 powder materials was obtained before the purification. Fig 1 compares the measured XRD peaks of the Hg2Br2 raw powder and ideal Hg2Br2 JCPDS peaks. (110) and (220) planes corresponding to the primary peaks of Hg2Br2 exactly matched the Hg2Br2 JCPDS peak, and other peaks such as (101) and (114) were also clearly detected. In order to check the binding energy states between the Hg and Br elements, XPS analysis was conducted, as shown in Fig 2. The orbital levels of Hg 4f5 and Hg 4f7, which are the main peaks of Hg element, were observed at 104.7 and 100.7 eV, respectively (Fig 2(a)). The binding energy of Br 3d was highlighted near 68.7 eV (Fig 2(b)). These results were consistent with the peak positions of Hg and Br reported in previous studies [17,18].
The composition ratio between Hg and Br was calculated to be approximately 2 : 1.5, indicating the Br element was relatively deficient with respect to the Hg atoms. The impurity of the raw powder should be monitored, since it may affect the quality of the finally grown Hg2Br2 crystal [13,19]. Thus, the impurities within the raw powder were detected using inductively coupled plasma-optical emission spectroscopy (ICP-OES) (Table 1). After excluding the detected impurity elements such as Fe, Ag, B, K, S and Si, the purity of the raw powder was calculated to be ~99.9913 %.
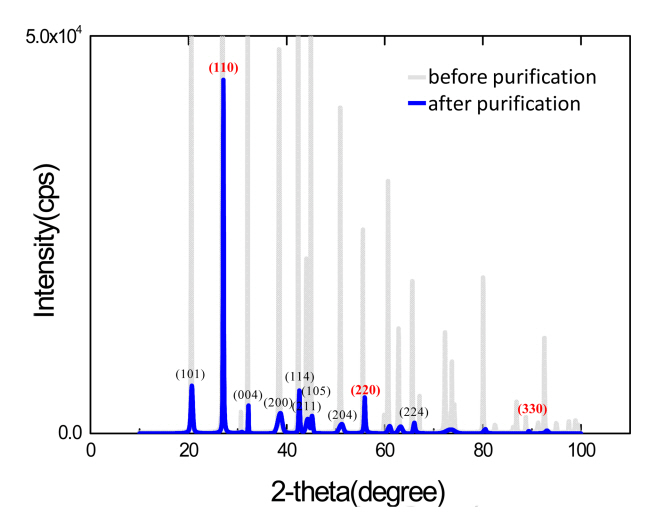
In order to obtain a higher purity powder, we proceeded with the purification of the Hg2Br2 powder using a PVT system. Figure 3 shows the XRD peaks of the Hg2Br2 powders before and after purification. Even though the associated peak intensity became smaller after the purification, the positions of the main peaks including (110) were almost same without considerable change. This indicates that the Hg2Br2 powder experienced no serious chemical reaction even after the purification process. In addition, the orbital binding energies of Hg 4f5, Hg 4f7, Br 3d peaks were featured at 103.6, 99.6, 67.7 eV, respectively, as shown in Figure 4.
A non-stoichiometric compound was made with a Hg : Br atomic ratio of 2 : 1.4. Inductively coupled plasma-mass spectroscopy (ICP-MS) analysis, which has a higher detection resolution than ICP-OES, was selected to detect impurities in the purified powder, as shown in Table 2. Impurity elements (Be, Ca, Co, Cu, Fe, Mo, Ti, Zn, Al, K, Ni, and Zr atoms) with a total concentration of 62 ppm were detected. Purity increased from a four-nine (99.9913%) to five-nine (99.99938%) material after the PVT process, which confirms it was an effective purification process.
The PVT process is normally suitable for the growth of Hg2Br2 single crystals because of the relatively low sublimation temperature of Hg2Br2 compared to its melting point. Thus, the PVT process was selected for the single crystal growth. A precisely controlled temperature gradient between the upper and bottom ampoule maintains the single crystal growth. For more effective temperature control of the ampoule, the PVT equipment was divided into three parts: a unit to control the process temperature or time, a driving unit to move the ampoule, and a chamber–heater unit around the ampoule. It was reported that delicate control of the temperature gradient within the ampoule highly influences the crystal quality [14].
Figure 5(a) shows Hg2Br2 crystals placed within the ampule during growth. The dimensions of the finally obtained crystals were ~35 mm in diameter and ~75 mm in length. The grown crystals were mechanically cut and polished for characterization. The main peaks in the (110) plane and its family (220) plane of Hg2Br2 crystals are shown in Fig 5(b). The 2-theta position and the full width at half maximum (FWHM) value of the (110) plane were 27.6o and 0.0677, respectively. This small FWHM indicates high crystallinity [13,20].
Figure 6 shows the chemical binder energy states of Hg and Br in the Hg2Br2 crystal, obtained by XPS analysis. To eliminate unintentional elements from the carbon contamination on the surface, the Hg2Br2 crystal was analyzed after a 90 nm etching process. The binding energy of Hg 4f5 and Hg 4f7 were detected at 104.6 and 100.6 eV, respectively, which is similar to the values obtained before powder purification (Fig 6(a)), while Br 3d peaks were also detected at 68.6 eV (Fig 6(b)). The relatively high intensity of the single Hg2Br2 crystal in the XPS analysis indicates high crystallinity compared with that from the purified powder, which clearly shows the doublet peaks of Br 3d originating from strong spin-orbit coupling. Using stoichiometry calculations, the composition ratio of Hg and Br in Hg2Br2 crystal was calculated to be ~2 : 1.76.
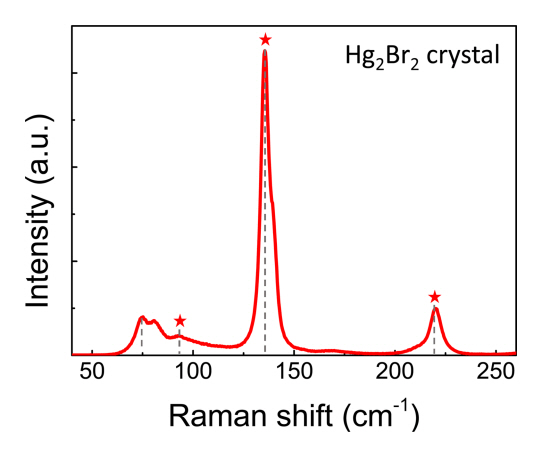
Raman spectra were analyzed to identify the lattice bonding in the Hg2Br2 crystal. According to group theory, the vibrational spectra of Hg2Br2 can be featured using four vibrational modes (35.5, 91, 136, and 221 cm−1) which can be divided into two groups: two doubly degenerate peaks (Eg of 35.5 and 91 cm−1) and two fully symmetric peaks (A1g of 136 and 221 cm−1) [21,22]. The rocking of the linear molecule and the zigzag bending vibrations of the Hg2Br2 amplified the Eg symmetry peak, and the Hg–Hg and Br–Hg stretching vibrations amplified the A1g symmetry peak.
As shown in Fig 7, all of the peaks related to the vibration mode of Hg2Br2 were detected, except for the 35.5 cm−1 peak, which was not detected within the measurement limits of the equipment. Meanwhile, extra peaks (75.2 and 80.8 cm-1) were observed, which do not match conventional Hg2Br2 crystals. The origin of these extra peaks should be further investigated.
4. CONCLUSION
In this study, we investigated the full PVT process, from purification to crystal growth, of Hg2Br2. The Hg and Br composition was confirmed using XPS analysis before and after purification. In the results of the XRD analysis, it was observed that the main 2-theta position of the powder was consistent with the JCPDS card of Hg2Br2. Based on ICP impurity analysis, the purity of the Hg2Br2 powder was increased from 4N (99.99%) to 5N (99.999%) through the purification process, which indicates its effectiveness. In addition, we characterized the Hg2Br2 crystal that was synthesized using purified powder. The FWHM values of the grown Hg2Br2 showed almost no lattice distortion in the crystal. The bonds constituting the Hg2Br2 lattice were identified from the Raman vibrational spectra of Hg2Br2 crystal. Establishing the full process sequence paves the way toward high quality AOM crystals, enabling promising AOTF modules.