1. INTRODUCTION
Lithium-ion batteries (LIBs) are now widely used in electric vehicles and other commercial applications [1,2]. Considering the demand for Li, Ni, and Co, recovering these metals from spent LIBs is of immense importance, and many hydrometallurgical recovery processes have been reported. In most, solvent extraction is employed to separate the metal ions from the spent LIB leaching solutions [3,4]. Several kinds of acidic and basic extractants, including D2EHPA, PC 88A, Cyanex 272, LIX 63, Alamine 336, and Aliquat 336, have been employed to separate metal ions from these inorganic acid leaching solutions [5-9].
In most acidic LIB leaching solutions, in addition to Co(II) and Ni(II), Cu(II) also exists. The presence of Cu(II) in the solution makes the separation process complicated. When Cu(II) is not completely separated from the solution, the purity of Co(II) and Ni(II) in the subsequent steps will be low and thus further purification steps will be needed to recover pure metal compounds. Therefore, separating Cu(II) is important to the overall effectiveness of metal recovery from spent LIBs [10].
Considering the high reduction potential of Cu(II), cementation methods can be employed for the separation of Cu(II). However, to recover Cu, the cemented Cu must be treated again. Conventionally, LIX 63 is widely employed for the solvent extraction of Cu(II) from acidic solutions. Meanwhile, other metal ions can also be extracted by LIX 63 at the same time Cu(II) is extracted [3].
Recently, we reported a process to separate Cu(II), Co(II), Fe(III), Mn(II), and Ni(II) from hydrochloric and sulfuric acid leaching solutions of spent LIBs [11]. In these processes, Cyanex 301 was employed for the separation of Cu(II) over the other metal ions. Although the separation factor between Cu(II) and other metal ions was very high, aqua regia had to be employed to strip Cu(II) from the loaded Cyanex 301. Considering that copper sulfate is employed for the manufacture of LIBs, it is desirable to find a new solvent extraction system which can be employed with a sulfuric acid solution as a stripping agent for Cu(II).
Several kinds of extractants have been employed for extracting Cu(II) [12-14]. Aliquat 336 shows selectivity for Cu(II) in moderate to strong acidic solutions, but the extraction performance is inferior to that of LIX 63 and Cyanex 301 [15]. Compared to a single extractant alone, higher extraction performance can be obtained by using a mixture of extractants [16-19]. Several mixtures of extractants have been reported for Cu(II) [19-22]. Considering the respective advantages of single LIX 63 and Aliquat 336 for the extraction of Cu(II), a mixture of these two extractants has some potential for the selective extraction of Cu(II).
The reduction smelting of spent LIBs at high temperature produces metallic alloys containing Co, Cu, Fe, Mn, Ni, and Si. These metals can be completely dissolved in a 2 M HCl solution containing H2O2 as an oxidizing agent [23]. Solvent extraction of the leaching solution with D2EHPA removes Fe(III), and the raffinate contains Co(II), Cu(II), Mn(II), Ni(II), and Si(IV) [24]. In this work, a mixture of LIX 63 and Aliquat 336 was employed and the extraction behavior of Cu(II), Co(II), Ni(II), Mn(II), and Si(IV) from the Fe(III) free raffinate was investigated under varying extraction conditions. A synergistic effect was observed for the selective extraction of Cu(II) from other metal ions. Moreover, it was possible to strip the Cu(II) from the loaded mixture with a weak sulfuric acid solution, which made it possible to recover copper sulfate from the stripping solution.
2. EXPERIMENTAL
2.1 Reagents and chemicals
Table 1 shows the composition of the Fe(III) free raffinate after solvent extraction with D2EHPA (Di-2-Ethylhexyl Phosphoric Acid, Co., 95%) from the HCl leaching solution of metallic alloys. The concentration of HCl in the Fe(III) free raffinate was 1.4 M. In this work, a synthetic HCl solution with the same composition as Table 1 was employed in the experiments. The synthetic solution was prepared by dissolving a certain amount of metal chloride in hydrochloric acid solution (HCl, Daejung Chemical & Metals Co., 35%, Korea). For this purpose, CoCl2┬Ę6H2O (Junsei Chemical Co., >97%, Japan), MnCl2┬Ę4H2O (Daejung Chemical & Metals Co., >98%, Korea), Na2SiO3 (Daejung Chemicals & Metals Co., Korea), NiCl2┬Ę6H2O (Yakuri Pure Chemicals Co., >96%, Japan), CuCl2┬Ę2H2O (Daejung Chemicals & Metals Co., >97%, Japan) were employed without any purification. In addition, hydrochloric acid and sulfuric acid (H2SO4, Daejung Chemical & Metals Co., >95%, Korea) were diluted with deionized water to obtain the desired concentrations.
The organic phases were prepared by mixing different ratios of Aliquat 336 (N-methyl-N, N, N-triethylammonium chloride, BASF Co., 93%, Germany) and LIX 63 (5,8-diethyl-7-hydroxydodecan-6-oxime, BASF Co., 70%) in commercial grade kerosene (Daejung Chemical & Metals Co., >90%, Ltd, Korea) and 1-decanol (Yakuri Pure Chemical Co., Ltd, Japan, >96%). Kerosene and 1-decanol were used as a diluent and a modifier, respectively. All organic reagents were used without any purification.
2.2 Procedure
Plastic screw cap bottles were used to hold equal volumes (20 mL) of aqueous and organic solutions in the extraction and stripping experiments. The two phases were shaken using a shaker (Burrell model 75, USA) for 30 min. The concentration of metal ions in the aqueous phase was measured by ICP-OES (OPTIMA 8300, Perkin Elmer) and that of the metal ions extracted into the organic phases was calculated by mass balance. All experiments were performed at room temperature.
3. RESULTS AND DISCUSSION
3.1 Effect of the mixture of Aliquat 336 and LIX 63 on the extraction of Cu(II)
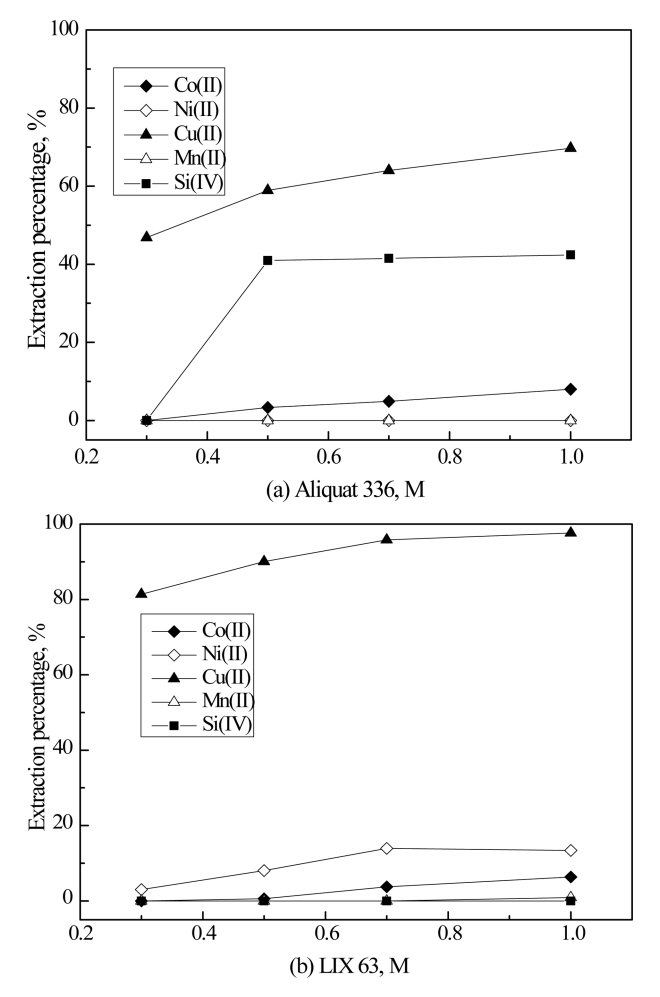
When the concentration of HCl is 1.4 M, some metal ions can form anionic complexes with chloride ions. In this case, Aliquat 336 can extract anionic complexes by anion exchange mechanism. With this in mind, solvent extraction experiments were carried out using single LIX 63 and Aliquat 336 to investigate the synergism of the mixture of LIX 63 and Aliquat 336 for the extraction of Cu(II). The concentrations of the two extractants were varied from 0.3 to 1.0 M and the results are shown in Fig 1. In Aliquat 336 diluted with kerosene, 10% v/v of 1-decanol was added as a modifier to prevent the formation of a third phase [21]. The extraction percentage of Cu(II) increased with the concentration of the two extractants, and the extraction percentages of Cu(II) by 1 M Aliquat 336 and LIX 63 were 69.7% and 97.6%. Meanwhile, 13.4% of Ni(II) and 6.3% of Co(II) were extracted by LIX 63, while 8.0% of Co(II) was also extracted by Aliquat 336.
Fig 1 clearly indicates that the two extractants have some advantages and disadvantages as an extractant for Cu(II) when they were employed individually at a concentration range below 1 M. Aliquat 336 shows selectivity for Cu(II) but the extraction percentage of Aliquat 336 for Cu(II) was lower than that by LIX 63. In contrast, the extraction percentage of Cu(II) by LIX 63 was high but Co(II) and Ni(II) were also co-extracted. Therefore, it can be anticipated that the mixture of Aliquat 336 and LIX 63 would show the combined advantages of the two extractants.
To investigate any synergism with the mixture of Aliquat 336 and LIX 63, mixtures of both extractants were employed, and the extraction results are shown in Fig 2. Fig 2(a) represents the extraction results for the mixture of 0.3 M Aliquat 336 and LIX 63 at varying concentrations from 0.1 to 0.5 M. This figure shows that a small amount of Ni(II) was co-extracted with Cu(II) when the concentration of LIX 63 exceeded 0.3 M in the mixture. Therefore, 0.2 M LIX 63 was considered to be the optimum concentration in the mixture for the selective extraction of Cu(II). Fig 2(b) shows the extraction results obtained by varying Aliquat 336 concentrations in the mixture with 0.2 M LIX 63. A slight increase in the percentage extraction of Cu(II) was observed with the increasing concentration of Aliquat 336 in the mixture. It is noticeable in Fig 2(b) that only Cu(II) was selectively extracted by the mixture, leaving Co(II), Ni(II), Mn(II) and Si(IV) in the raffinate. This confirms that it was possible to separate Cu(II) from the Fe(III) free raffinate. Specifically, 90% of Cu(II) was selectively extracted over other metal ions by the mixture of 0.3 M Aliquat 336 and 0.2 M LIX 63.
In solvent extraction with a mixture of two extractants, the synergistic coefficient is defined as follows:
where D represents the distribution ratio and R is the synergistic coefficient. Dmix,Cu, DA,Cu, DL, Cu are the distribution ratios of Cu(II) in the mixture, single Aliquat 336, and single LIX 63, respectively;
Table 2 shows the data on the distribution ratios and synergistic coefficients of Cu(II) for the single Aliquat 336 and LIX 63, and for the mixture of these two extractants. With the mixture, in most of the extraction conditions the values of R were higher than unity, indicating the mixture of Aliqaut 336 and LIX 63 has a synergism for the extraction of Cu(II). The highest synergistic coefficient of 4.28 was obtained for the extraction of Cu(II) when the concentration of Aliquat 336 and LIX 63 in the mixture was 0.3 M and 0.2 M. Since only Cu(II) was selectively extracted from the solution by this mixture, the mixture of 0.3 M Aliquat 336 and 0.2 M LIX 63 was employed in the subsequent experiments.
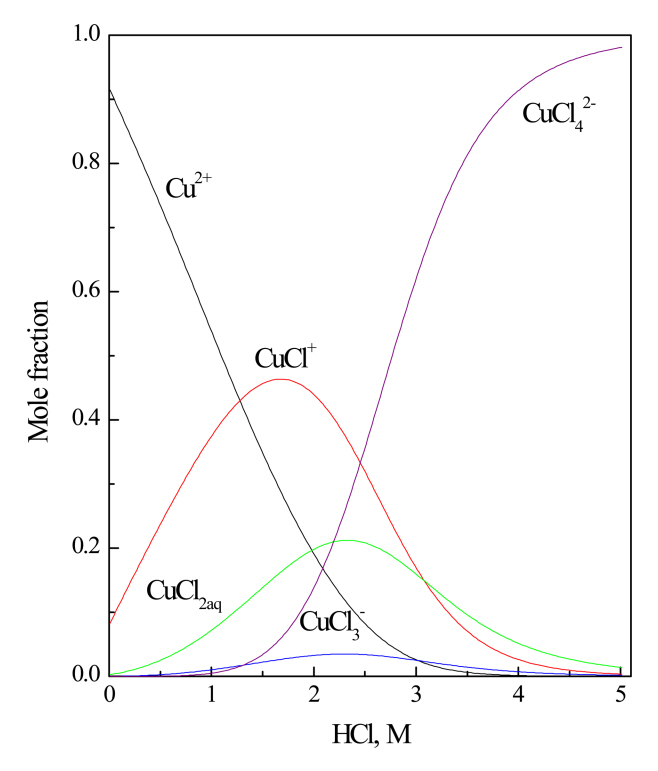
Fig 3 shows the speciation diagram of Cu(II) in HCl solution [25]. When the concentration of HCl is 1.4 M, Cu(II) exists as a cation and anionic complex like Cu2+ and CuCl3-, respectively. Therefore, with the mixture of Aliquat 336 and LIX 63 the extraction reaction of Cu(II) can occur by both cation and anion exchange, as represented in the following equations [26,27].
In the above equations, HA and R4NCl represent LIX 63 and Aliquat 336, respectively. As represented in Fig 3, the speciation of Cu(II) depends on HCl concentration. Therefore, HCl concentration would affect the extraction performance of the mixture of Aliquat 336 and LIX 63 for Cu(II). Considering the characteristics of the cation and anion exchange reaction, an increase in HCl concentration would favor the extraction of Cu(II) by Aliquat 336 but suppress that by LIX 63 [28]. In order to verify this expectation, the concentration of HCl in the solution was reduced from 1.4 M to 0.1 M. In these experiments, the concentrations of Aliquat 336 and LIX 63 in the mixture were fixed at 0.3 and 0.2 M, respectively. Fig 4 shows that the extraction percentage of Cu(II) was reduced from 90.4% to 67.9% as the HCl concentration was decreased from 1.4 M to 0.1 M. The speciation diagram of Cu(II) shows that the mole fraction of CuCl3- decreased as the HCl concentration was reduced from 1.4 M to 0.1 M. Therefore, the contribution of Aliquat 336 to the extraction of Cu(II) would be reduced as the HCl concentration decreased. It should be noted that there is no need to adjust the pH of the solution for the extraction of Cu(II) using the mixture of 0.3 M Aliquat 336 and 0.2 M LIX 63.
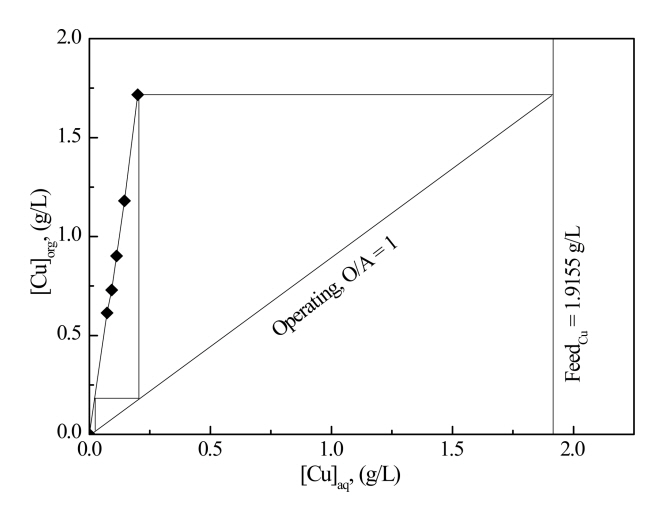
Fig 5 is the McCabe-Thiele diagram for the extraction of Cu(II) using the mixture of 0.3 M Aliquat 336 and 0.2 M LIX 63. This figure shows that more than two stages of counter-current extraction are required to completely extract Cu(II) from the solution with the mixture of 0.3 M Aliquat 336 and 0.2 M LIX 63. Table 3 shows the extraction percentage of the metal ions while varying the volume ratio of the two phases. This table shows that the extraction percentage of Cu(II) increased slightly with the increase in the volume ratio of the organic to aqueous phase. When the volume ratio of organic to aqueous was 3, the extraction percentage of Cu(II) was 96.2%. However, Co(II), Ni(II) and Si(IV) were also extracted when the volume ratio of organic to aqueous was higher than 2. Therefore, for the selective extraction of Cu(II) from the solution, it is important to adjust the volume ratio of organic to aqueous to unity.
In continuous experiments employing a mixer-settler, the flow rate of the two phases is related to the residence time in the mixer. Since the flow rate of the two phases depends on the reaction kinetics, the effect of reaction time on the extraction was investigated. Fig 6 clearly shows that the extraction kinetics of Cu(II) using the mixture was very fast, and the reaction time did not affect the extraction percentage of Cu(II) within 30 min.
Considering the fast reaction kinetics and high extraction percentage of Cu(II), the mixture of LIX 63 and Aliquat 336 can be employed for the selective extraction of Cu(II) from solution in industrial operations.
3.2 Stripping of Cu(II) from the loaded mixture by sulfuric acid and thiourea solution
When the mixture of 0.3 M Aliquat 336 and 0.2 M LIX 63 was employed for the extraction of Cu(II) from the Fe(III) free raffinate, the concentration of Cu(II) in the loaded organic phase was 931.2 mg/L. To strip the Cu(II) from the loaded organic, a sulfuric acid and thiourea were employed as stripping agents and the two phases were shaken for 30 min [29]. In these experiments, the concentration of sulfuric acid and thiourea was varied. The stripping results at the same volume ratio of the two phases are shown in Table 4.
In the case of stripping with sulfuric acid, the stripping percentages of Cu(II) by 0.1 M and 0.5 M sulfuric acids were 90 and 91%, respectively. However, the Cu(II) stripping percentage by 3 M sulfuric acid was 58%, indicating that sulfuric acid concentration has a negative effect on the stripping of Cu(II) from the loaded mixture. Since stripping is the reverse reaction of extraction, these stripping results were in good agreement with the extraction data. That is, the stripping percentage of Cu(II) would decrease with increasing sulfuric acid concentration.
Meanwhile, the stripping percentage of Cu(II) by thiourea was 89%, irrespective of its concentration in the range from 0.1 to 3 M. When pure thiourea solution was employed as a stripping agent, white colloids were observed in the stripping solution. This may be related to the pH of the stripping solution. When a pure thiourea solution was employed, the stripped Cu(II) was precipitated because of the higher pH of the solution. Therefore, some acid should be added to the thiourea solution to prevent the precipitation of the stripped Cu(II).
When the concentration of sulfuric acid as well as thiourea was below 0.5 M, the percentages of Cu(II) stripping by these two agents were similar. Considering the price, sulfuric acid can be selected as a stripping agent for Cu(II) from a loaded mixture. Therefore, 0.1 M H2SO4 was considered to be the optimum concentration for the stripping of Cu(II). Fig 7 shows the McCabe-Thiele diagram for the stripping of Cu(II) by 0.1 M sulfuric acid from the loaded mixture. Three stages of counter-current stripping were required to completely strip Cu(II) from the loaded mixture.
The McCabe-Thiele diagrams for the extraction and stripping of Cu(II) using the mixture of extractants and sulfuric acid indicate the number of extraction and stripping stages (see Figs 5 and 7). In order to verify the complete extraction and stripping of Cu(II), batch simulation experiments for the counter-current extraction and stripping were carried out and the results are shown in Table 5. In the three stages of counter-current extraction, 99% of Cu(II) was extracted into the mixture of 0.2 M LIX 63 and 0.3 M Aliquat 336, but other metal ions remained in the raffinate. Therefore, it was possible to selectively extract Cu(II) from the solution. After three stages of batch simulation counter-current stripping experiments, about 4 ppm of Cu(II) was not stripped, indicating that at least 4 stages are required to completely strip the Cu(II) from the loaded mixture. Since only Cu(II) is in the stripping solution, the purity of Cu(II) in the stripping solution was very high. Therefore, extra pure copper sulfate can be recovered from the stripping solution.
Table 6 compares the separation efficiency of Cu(II) in our previous work [11] and in the current work. When single Aliqaut 336 was employed as an extractant for Cu(II), a small amount of Co(II) was co-extracted and thus a subsequent separation step was necessary to obtain pure Cu(II) solution. When Cyanex 301 was used as an extractant, only Cu(II) was extracted into the organic, but aqua regia was needed to strip the Cu(II) from the loaded Cyanex 301, because of the strong interaction between Cu(II) and Cyanex 301. In contrast, it was possible to selectively extract Cu(II) from the solution using the mixture of LIX 63 and Aliquat 336. Additionally, a weak sulfuric acid solution can strip the Cu(II) from the loaded mixture.
Our work indicates that the mixture of LIX 63 and Aliquat 336 is superior to single Aliquat 336 or Cyanex 301 as an extractant for Cu(II) in terms of selectivity, extraction and stripping performance. Some more work needs to be done to test the performance of the mixture during long time operation. Using the mixture for batch simulation extraction and Cu(II) stripping, a high purity CuSO4 stripping solution and Cu(II) free raffinate were obtained, leaving Co(II), Mn(II), Ni(II) and Si(IV) in the raffinate. Extra pure CuSO4 can be obtained from the stripping solution and the metal ions in the Cu(II) free raffinate can be further separated using our reported processes [24].
4. CONCLUSIONS
In this work, solvent extraction experiments were performed to separate Cu(II) from the HCl solution containing Co(II), Mn(II), Ni(II), and Si(IV). For this work, a mixture of Aliquat 336 and LIX 63 was employed as the extractant. Our results indicated that this mixture could selectively extract Cu(II) from other metal ions. Only Cu(II) was extracted from the solution when the concentrations of Aliquat 336 and LIX 63 were 0.3 M and 0.2 M, respectively. Moreover, the mixture showed a synergism for the extraction of Cu(II) from the HCl solution. The highest synergistic coefficient of 4.28 for the extraction of Cu(II) was obtained using mixture of 0.3 M Aliquat 336 and 0.2 M LIX 63. Weak sulfuric acid solutions were able to completely strip the Cu(II) from the loaded mixture. McCabe-Thiele diagrams for the extraction and stripping of Cu(II) by the mixture were constructed. Batch simulation experiments for the counter-current extraction and stripping indicated that only Cu(II) was completely extracted, and the purity of Cu(II) in the stripping solution was higher than 99.9%. This work introduces a solvent extraction process to recover Cu(II) with high purity from the HCl leaching solution of spent LIBs.