Continuous Synthesis of Monodisperse Spherical Silica Powder Using Tubular Reaction System
Article information
Abstract
In the present study, monodisperse silica nanospheres were synthesized using a tubular reaction system in a continuous way. Screw-type blades were inserted inside a T-mixer for static mixing of the reactant streams which consisted of TEOS and NH4OH/H2O diluted with ethanol, for the continuous synthesis of the silica suspension. The diameter of the silica powder was monitored as a function of production time using dynamic light scattering to determine the optimum retention time and tube length, which were found to be 125 minutes and 7.5 m, respectively. The effects of reactant compositions on particle size were investigated by adjusting the amount of ammonia and water in the sol-gel reaction, which were then compared with the results from a batch reactor. Both the particle size and polydispersity index (PDI) of the silica suspension were measured to be comparable to nanospheres synthesized using a batch reactor. This implies that the tubular reaction system is more beneficial for potential industrial production applications in the size range from 115 to 310 nm, due to its continuous powder synthesis. The effect of the reaction medium was also studied, by replacing ethanol with methanol or propanol, indicating that the deviation in particle size with production time was not a serious issue in alcohols with lower molecular weight, such as methanol and ethanol.
1. INTRODUCTION
The synthesis of monodisperse particles such as polymeric latex or ceramic beads has been considered as an important research topic in the field of colloid science and nanotechnology. Specifically, metal oxide particles with narrow size distribution have been studied for various applications such as building block materials of self-assembly, cosmetics, chemical mechanical planarization, diagnostics of medical applications, and rheological studies of complex fluid system [1-6].
Among various colloidal dispersion systems composed of monodisperse particles, spherical silica suspension has been most intensively studied due to facile nature of the synthesis reactions and easy control of the particle diameter under different reaction conditions. Though silica particles have been synthesized in various morphologies including hollow particles, simple spherical shape can be prepared by the most simple manner [7]. Since the precursor materials of silica particles such as tetraethylorthosilicate (TEOS) usually undergoes relatively slow rate of hydrolysis reaction compared to the other precursor materials such as titania or zirconia, fine control of the sol-gel reaction has been accomplished by Stober [8]. Further improvements on the Stober synthesis have been also reported by the other research groups for the possible application of the monodisperse silica particles to colloidal photonic crystals [9]. Additionally, the in-depth study on the characteristics of Stober reaction was carried out by Giesche from the series of batch reactions to determine the rate constant [10]. From the detailed research, the first order reaction kinetics was found from the synthesis reactions of Stober silica, and microporous nature of the silica particles could be examined using BET adsorption and desorption results.
Usually, the synthesis of monodisperse particles has been carried out using laboratory-scale batch-type reactor to study the factors affecting the particle size and distribution. However, batch type reactors have drawbacks in that it is difficult to establish the scale-up conditions for large-scale production of particle suspensions. Thus, it is essential to study the continuous production of monodisperse colloids using proper reaction systems such as tubular reactor. It is still challenging to perform the systematic researches on the synthesis of silica nanospheres using tubular reactors in controlled manners, especially for the potential applications of colloidal particles to various industrial applications. Though the continuous synthesis of silica particles using tubular reactor has been carried out by Kimata and his colleagues recently, their results are limited to the nanoparticles smaller than 100 nm and their researches were mainly performed using methanol as reaction medium [11]. Thus, additional systematic researches are necessary, especially for the processing parameters such as the retention time, reactant compositions, and mixer configurations for the synthesis of monodisperse silica articles larger than 100 nm.
In this study, monodisperse silica nanospheres were synthesized by tubular reactor for the continuous production of the particles. For sol-gel reaction, silica precursor such as TEOS (tetraethylorthosilicate) and ammonia/water mixture were separately fed to the T-mixer which connects two input flows of the reactants into one outlet tube composed of Teflon. The mixing of the reactants was performed using static mixer for the complete hydrolysis and condensation reaction of TEOS. For the size control of the particles, retention time of the reactants was adjusted by changing the length of the tubular reactor or feeding rate of the liquid materials. The reactant composition was also changed to study the effect of the particle size and distribution of the silica nanospheres and the results were compared with the silica nanospheres produced from batch-type reactor. The resultant silica nanospheres were characterized by particle size analyzer and scanning electron microscope to measure the particle diameter and monodispersity.
2. MATERIALS AND METHODS
2.1. Materials
Tetraethylorthosilicate (TEOS, 99%, Aldrich) was used as the precursor material of silica nanospheres. Aqueous NH4OH solution (28-30%, Samchun Chemicals) was adopted as catalyst for the hydrolysis and condensation reaction and ethanol (HPLC grade, Burdick & Jackson) was used as reaction medium. De-ionized water (18.2 MΩcm-1) was used for the hydrolysis of TEOS and generated by using distilled water system (direct Q-3 with pump, Millipore).
2.2. The synthesis of monodisperse silica nanospheres using batch-type reactor
Silica nanospheres were synthesized using TEOS as silica source material and NH4OH as catalyst of sol-gel reaction. Ethanol was used as reaction medium and water was added for hydrolysis of TEOS under vigorous stirring inside roundbottom flask during Stober reaction. The reactant compositions summarized in Table 1 was usually adopted for the silica synthesis for the reaction time of 2 hours. Sometimes, the reactant compositions such as the amount of TEOS or reaction time were adjusted to observe the change of average particle size and size distribution.
2.3. The synthesis of monodisperse silica nanospheres using tubular reactor
Typically, monodisperse silica nanospheres were synthesized using tubular reactor with continuous flow streams of reactants. The reactants such as TEOS dissolved in ethanol and the mixture of NH4OH/H2O diluted with ethanol were fed to the two input parts of T-mixer (Teflon, Larminar co. ltd). The reactants were mixed inside T-mixer by the mechanism of static mixing using screw-type blades (7 cm in length, unmounted type, polyacetal, Coleparmer) and the particle suspension was synthesized by sol-gel reaction. The diameter of the cross-section of the static mixer was 6 mm, and pitch of the mixer was also measured as 6 mm. Then, monodisperse silica nanospheres were continuously injected out of tubular reactor (ID = 2 mm) made of Teflon connected with the T-mixer. The length of tubular reactor was fixed as 5, 6.25, or 7.5 m and the retention time was adjusted by controlling the feeding rate of the reactant flow streams using syringe pump (NE-4000, New Era, USA). The particle suspension was collected with the time interval of 20 minutes after the colloidal dispersion of silica nanospheres was first injected outside of the tubular reactor.
2.4. Characterizations
The particle size and distribution were measured by particle size analyzer (ZETA PLUS, Marlvern Instruments). The morphologies of the silica particles were observed using field emission scanning electron microscope (FE-SEM, Hitachi-S4700).
3. RESULTS AND DISCUSSION
In this study, simple test reactions with batch-type reactor were performed to determine the proper reactant compositions for the synthesis of monodisperse silica nanospheres. The reactant solution became opaque within a few minutes due to the generation of nucleus of silica particles during Stober reaction. The hydrolysis and condensation reaction of TEOS and water with ammonia catalyst can proceed under the following reaction scheme.
Figure 1(a) contains the average size of silica particles synthesized using batch-type reactor with varying amount of TEOS. As the volume percent of TEOS increases, the size of silica particles increased monotonically, since increase of silica source material such as TEOS will cause the enlargement of the resultant silica nanospheres. However, the polydispersity index (PDI) of silica particles revealed optimum value such as 0.07 when 5.83 vol. % of TEOS was used in sol-gel reaction, as displayed in the graph of Figure 1(b). Thus, the composition of reactants was determined based on the optimum value of TEOS with minimum PDI for the synthesis of monodisperse silica nanospheres using continuous tubular reactor. The typical composition for silica synthesis using tubular reactor is summarized in Table 2.
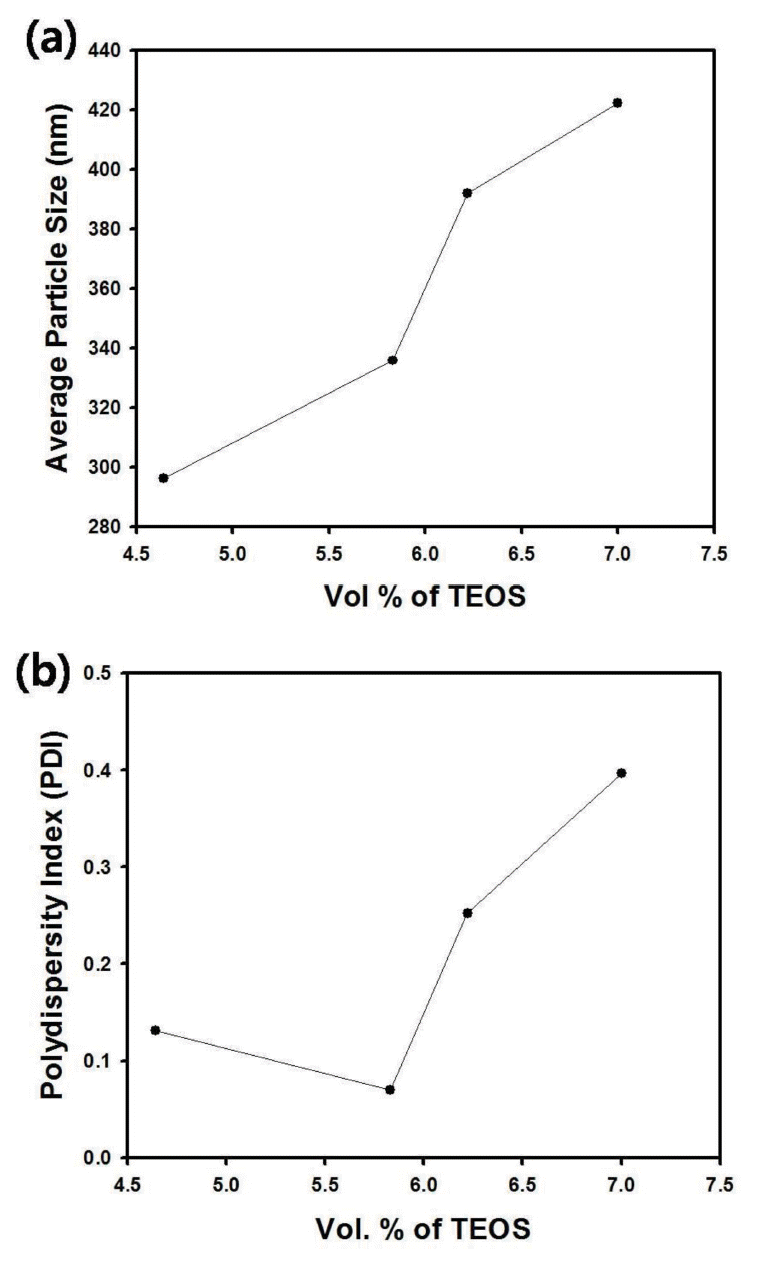
(a) Average size and (b) polydispersity index (PDI) of silica particles synthesized using batch-type reactor as a function of amount of TEOS.

Typical synthesis conditions of silica nanospheres using tubular reactor as continuous production system.
Figure 2(a) contains the schematic figure of tubular reactor as continuous system for the synthesis of monodisperse silica nanospheres. There are two reactant streams such as TEOS diluted with ethanol and water/ammonia mixture dissolved in ethanol fed to T-mixer by syringe pump. The reactants are induced to be mixed intimately by passing through the blades inside the T-mixer which is connected with Teflon tube with at least 5 m in length. The long tubular reactor is intended to keep sufficient retention time of the liquid materials during sol-gel reaction for the synthesis of monodisperse silica particles. The eluted silica suspension was taken for the characterization of particle size analysis for every 20 minutes. Figure 2(b) contains the ‘configuration 1’ of mixing system, which is composed of one or two blade(s) inserted in T-mixer connected to the inlet part of the Teflon tube. Through this system, the reactant streams were mixed in the early stage of reaction before passing through the tubular reactor. The T-mixer was also composed of Teflon with the structure shown in Figure 2(c) to avoid the material damage from various chemicals.
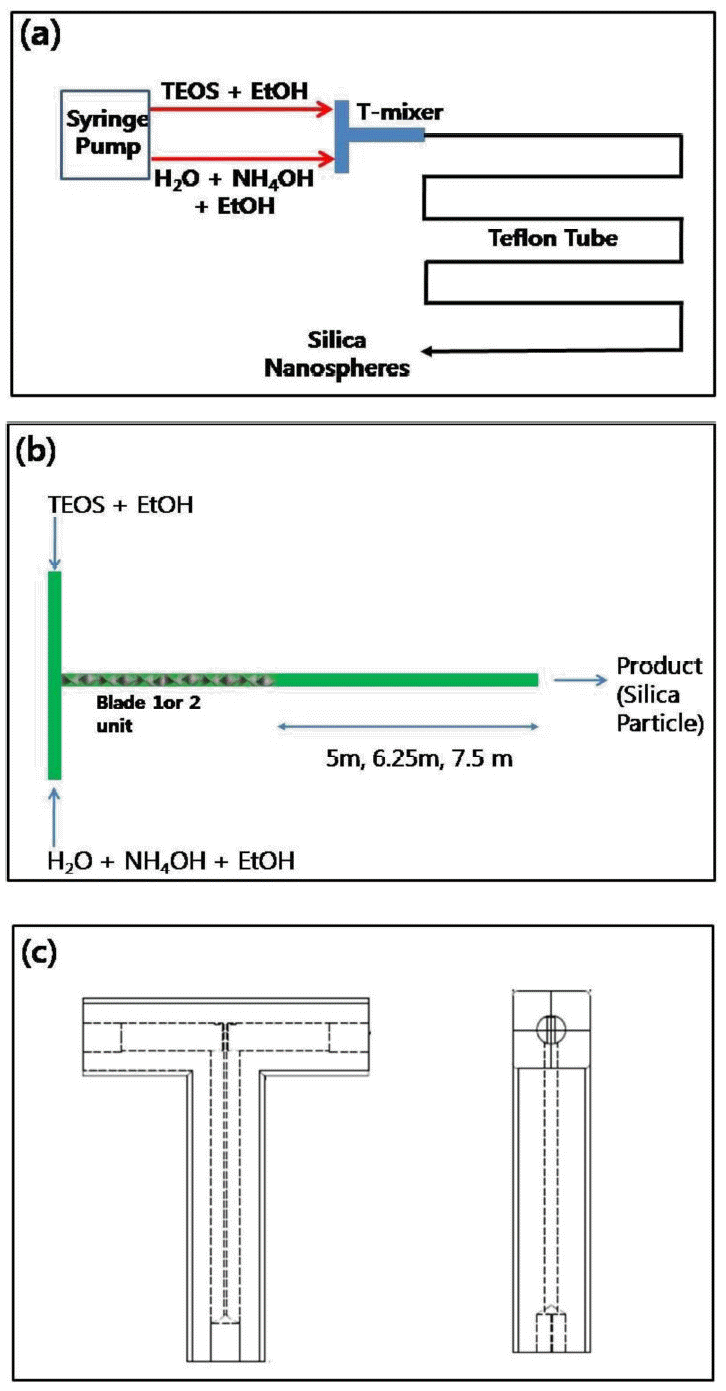
(a) Schematic figure for the fabrication of monodisperse silica nanospheres using tubular reactor with continuous flow streams. (b) Schematic figure of the configuration of T-mixer with mixing units (blades) attached to the inlet position of Teflon tube. (c) The structure of T-mixer used in this study.
Figure 3(a) contains the average size of the silica nanospheres as a function of production time. The size of the particle suspension was measured by light scattering method using ZETA PLUS apparatus and retention time was adjusted from 68 to 162 minutes for the tubular reactor with 7.5 m in length. As displayed in the graph of Figure 3(a), the particle size fluctuates as a function of production time for the short retention time such as 68 minutes, which may not be enough for the completion of the reaction. However, the particle size was maintained with relatively uniform values with increasing production time when the retention time was longer than 68 minutes, implying that it requires sufficient retention of the reactants inside the tubular reactor for the completion of reaction. As retention time increased from 125 to 162 minutes, the size of silica particles increased from about 150 to 250 nm, as displayed in the graph of Figure 3(a).

(a) Average particle size of silica nanospheres as a function of production time. Retention time was controlled from 68 to 162 minutes. (b) Average particle size of silica nanospheres as a function of retention or reaction time for tubular or batch-type rector, respectively. The production time was fixed as 0 minutes for tubular reactor.
The solid line of Figure 3(b) indicates the average diameter of the silica particles as a function of retention time when the production time was fixed as 0 minute, just after the first elution of the particle suspension out of the tubular reactor. As shown in the solid line graph of Figure 3(b), the average particle size increased as a function of retention time, since the reaction conversion increases with increasing retention time of the reactants inside the tubular reactor, resulting in the enlargement of the particle diameter. For comparison, the average particle size of silica nanospheres as a function of reaction time is also plotted as dotted line in Figure 3(b) for batch-type reactor. The size enlargement of silica particles was also observed for batch-type reactor with larger particle diameter compared to silica nanospheres produced from tubular reactor, possibly due to the shear-induced aggregation by mechanical stirring in batch reactor [12]. Since the mixing mechanism of tubular reactor is static mixer type, strong shear force is not accompanied during the synthesis of silica particles, resulting in the smaller particle diameter compared to batch-type reactor.
Figure 4(a) contains the effect of tube length on the average particle size of the silica suspension. The injection rates of the reactant feeds were fixed as 85 µl/min and the tube length was changed from 5 to 7.5 m. The samples taken from the outlet of the tubular reactor were characterized by particle size analyzer and the average size of the silica nanospheres were compared each other when the production time was varied from 40 to 60 minutes. From the graph in Figure 4(a), the diameter of the particles taken in the production time of 40 and 60 minutes does not coincide with each other for the length of the tubular reactor with 5 and 6.5 m. However, the size of the particles in different production time seemed to be in the similar range for the tube length of 7.5 m with about 250 nm in diameter. Since sufficient retention time induces the saturated growth of the particles regardless of the production time, the synthesis of silica nanospheres with uniform diameter could be accomplished by tubular reactor with enough tube length such as 7.5 m. Thus, the tubular reactor with 7.5 m in length was adopted in the continuous flow synthesis system in this study. Figure 4(b) contains the scanning electron microscope image of silica nanospheres synthesized using tubular reactor with 7.5 m in length for the production time of 40 minutes. As can be confirmed by comparing Figure 4(a) and Figure 4(b), the size of the particles from the microscope image is a little bit smaller than the diameter of the particles measured using particle size analyzer, possibly due to the aggregation of the particles during light scattering.
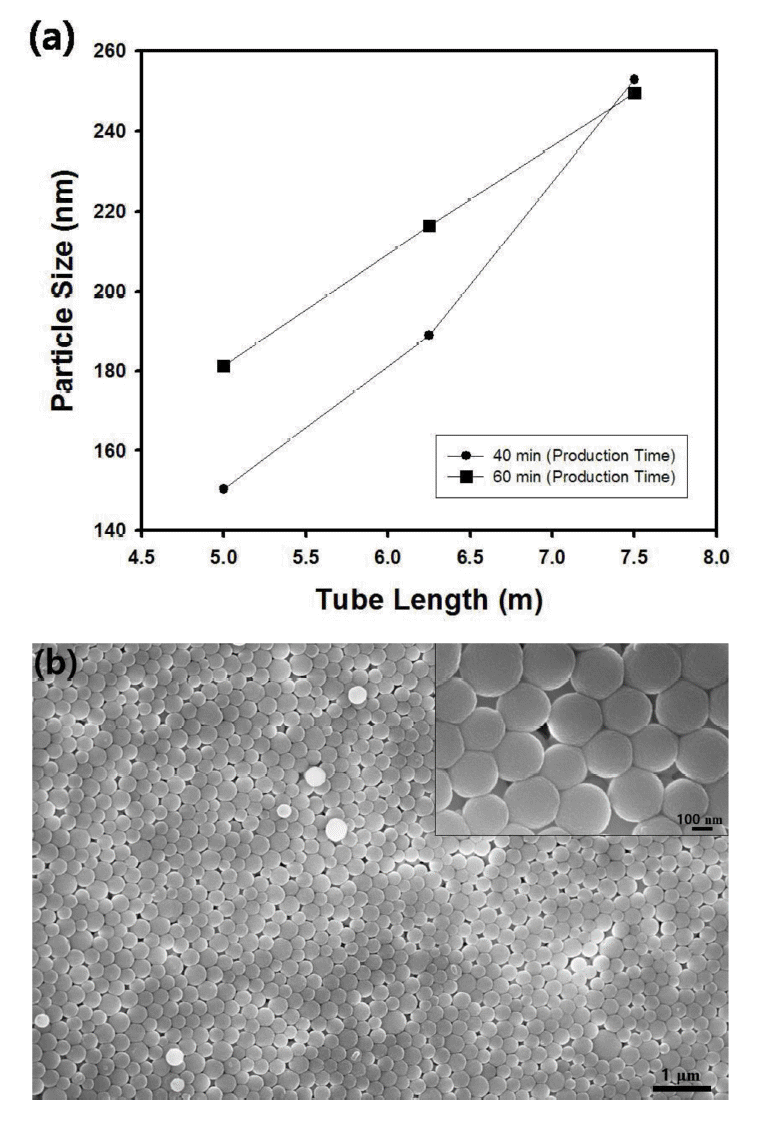
(a) Average particle size of silica nanospheres as a function of tube length. The injection rate of feed streams was fixed as 85 µ/min. (b) Scanning electron microscope image of silica nanospheres synthesized using tubular reactor with 7.5 m in length. The injection rate of feeds was 85 µl/min and the production time was 40 minutes.
Besides the retention time and tube length of the continuous production system, the reactant compositions were also adjusted to examine the variation of the diameter and size distribution of silica particles. Figure 5(a) displays the average particle size of the silica nanospheres as a function of production time with different ammonia concentration in the feed stream. Since ammonia acts as catalyst for the Stober reaction, the increased amount of ammonia caused the enlargement of the silica particles as displayed in the graph of Figure 5(a). The solid line of Figure 5(b) indicates the average particle size of silica nanospheres as a function of ammonia concentration for the samples taken in the production time of 20 minutes using tubular reactor. During this stage of the continuous reactor, the size of silica particles increased from 127.4 to 274.8 nm with increasing amount of ammonia due to the increase of the catalyst material. Similar trend was also observed from the batch-type reactor, as displayed in the dotted line of Figure 5(b). However, the synthesis of silica particles using batch-type reactor was more sensitive to the amount of ammonia compared to the case of tubular reactor, and the size of silica nanospheres could be controlled from 138.8 to 428.6 nm. Shear-induced aggregation may be responsible for the larger size of silica particles synthesized from batch-type reactor since the mixing of reactants is usually performed by mechanical stirring in such reactors [12]. On the contrary, the size of silica nanospheres was measured as relatively smaller values for the case of tubular reactor, and the polydispersity index (PDI) was maintained as smaller values than 0.08, implying that the size monodispersity is much better than that of silica particles from batch-type reactor, as displayed in the graph of Figure 5(c). Thus, the tubular reactor in this study can be applied as effective continuous synthesis system of monodisperse silica particles. On the contrary, batch-type reactor has drawbacks in that polydisperse particles can be synthesized under the conditions such as serious vibration of the liquid meniscus during mechanical stirring and scale-up may be a difficult issue for large production of monodisperse silica suspension.
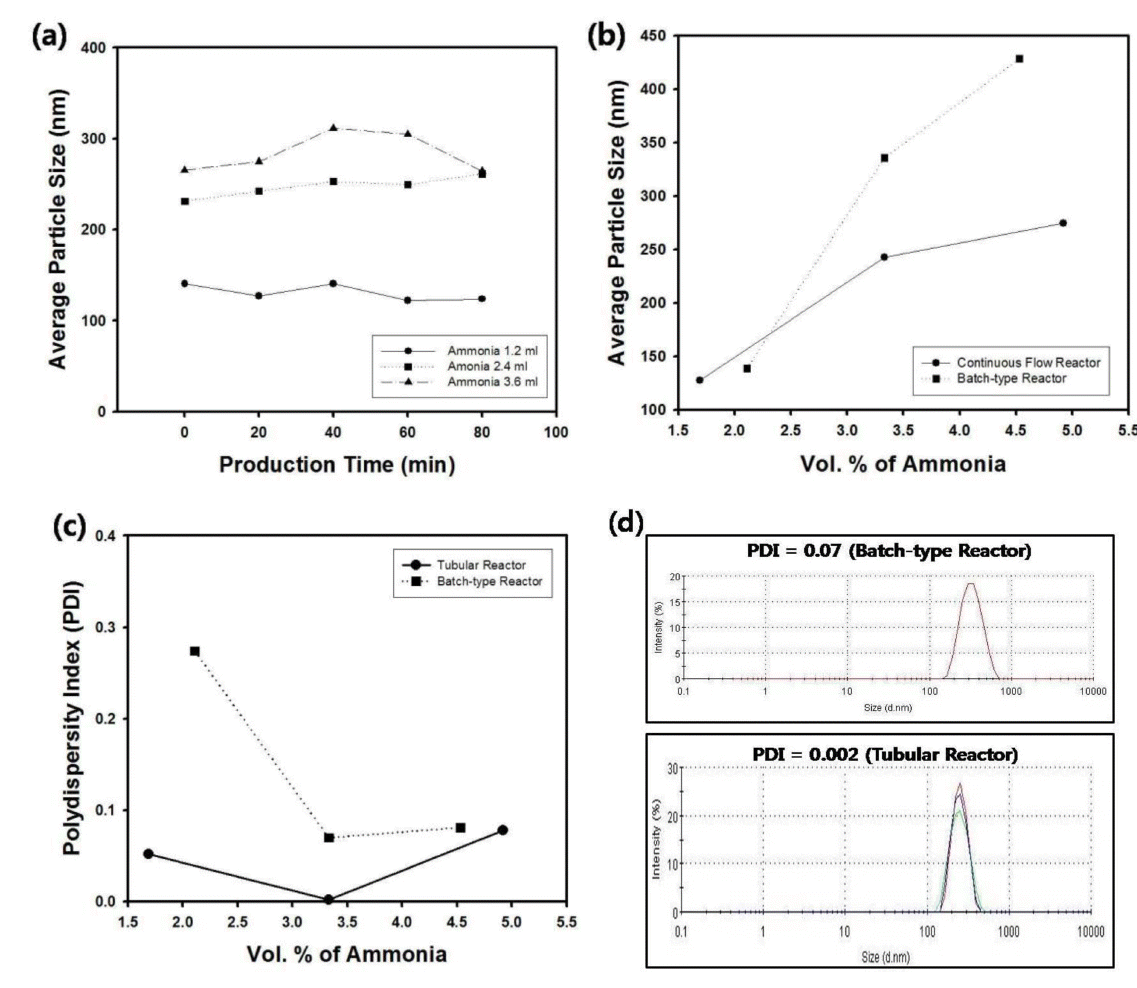
(a) Average particle size of silica nanospheres synthesized using tubular reactor as a function of production time. The amount of ammonia was controlled from 1.69 to 4.92 vol. %. (b) Average particle size and (c) polydispersity index (PDI) of silica nanospheres as a function of ammonia concentration. The dotted line indicates the data from batch-type reactor. For the data from tubular reactor, the production time and injection rates of reactants were fixed as 20 minutes and 85 µl/min, respectively. (d) Size distribution of silica nanospheres synthesized using batch-type and tubular reactor. For the batch-type reactor, the amount of ammonia was fixed as 3.3 vol. %. For the data from tubular reactor, the production time and injection rates of reactants were fixed as 20 minutes and 85 µl/min, respectively.
From Figure 5(c), it is evident that there exists optimum value of ammonia concentration for the PDI of silica particles for both batch-type and tubular reactor, since proper amount of ammonia promotes electrical charge on the particle surfaces to avoid the flocculation. When the concentration of ammonia was too high, PDI of silica particles increased possibly since the formation of secondary nuclei can be promoted by the excessive amount of catalyst. Figure 5(d) contains the particle size distribution of silica nanospheres synthesized using batch-type and tubular reactor, which corresponds to the data points shown in the graph of Figure 5(b) and Figure 5(c) with the volume fraction of ammonia as 3.3 %. Under the same reactant compositions, the particle size distribution of the produced silica nanospheres from tubular reactor is more monodispersed compared to that of silica particles obtained using batch-type reactor.
The particle size distribution of silica nanospheres from tubular reactor displayed in Figure 5(c) is quite monodisperse but not perfectly mono-sized. This can be explained by the flow distribution of liquid materials inside Teflon tube with low Reynold number regime subject to non-slip boundary condition on the tube wall. The parabolic velocity profile of the reactant streams inside tube causes the differences of residence time with relatively long or short residence time near tube wall or tube center, respectively [13]. Thus, the distribution of reaction conversion along tube radius will generate the silica nanospheres with size distribution as well. However, by adjusting the operation conditions and reactant compositions, the PDI value of the particles can be reduced lower than 0.005, as shown in the graph of Figure 5(c).
Figure 6(a) contains the average particle size of silica nanospheres as a function of production time with varying amount of water concentration in the reactant feed stream to the T-mixer. The amount of water was controlled from 5.13 to 9.76 vol. %, and the injection rates of reactants were fixed as 100 µl/min. The increase of water concentration resulted in the formation of large silica particles due to the enhanced rate of hydrolysis reaction in the sol-gel process. When the production time was fixed as 0 minute (just after the start-up of the particle production), the size of silica suspension increased linearly as a function of water concentration, as displayed in the solid line of Figure 6(b). For comparison, the data from batch-type reactor was also displayed as dotted line in Figure 6(b), indicating that the particle size is larger than the samples produced by tubular reactor, possibly due to the shear-induced coagulation during mechanical agitation of the reactants. The polydispersity index (PDI) of silica nanospheres was also compared for batch-type and tubular reactor, and relatively monodisperse particles could be produced from tubular reactor with continuous flow streams. When the concentration of water was too high, PDI of the silica particles increased since the formation of new nuclei results in the polydisperse particle system.
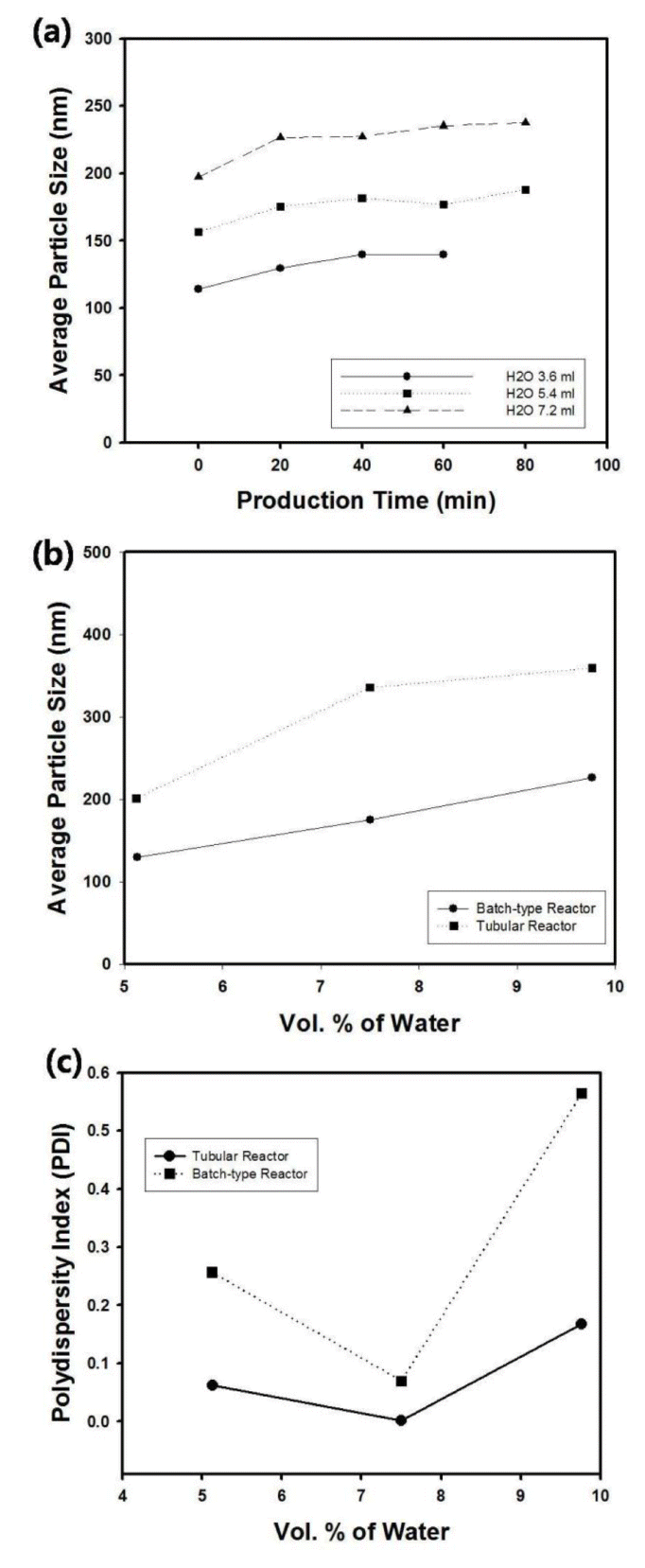
(a) Average particle size of silica nanospheres synthesized using tubular reactor as a function of production time. The amount of water was controlled from 5.13 to 9.76 vol. %. (b) Average particle size and (c) polydispersity index (PDI) of silica nanospheres as a function of water concentration. The dotted line indicates the data from batch-type reactor. For the data from tubular reactor, the production time and injection rates of reactants were fixed as 0 minutes and 200 µl/min, respectively.
Figure 7(a) contains the change of average particle size of silica nanospheres as a function of the amount of TEOS fed to the tubular reactor with 7.5 m in tube length. The reactants streams were fed to the T-mixer with the injection rate of 100 µl/min and TEOS concentration was controlled from 4.24 to 7.44 vol. %. Since the size of silica particles increased with increasing concentration of precursor materials such as TEOS, the trend was similar with the results from batch-type reactor, as displayed in the graph of Figure 7(a). However, the average size of the silica particles was smaller than the diameter of silica suspension obtained from batch reactor possibly due to the absence of shear-induced aggregation. Figure 7(b) contains the change of the particle size of silica suspension as a function of production time with different concentration of TEOS. This graph indicates that the size of silica particles increased with production time under the low concentration of TEOS, whereas the diameter of silica nanospheres was maintained as almost constant value when the concentration of TEOS was relatively high. Thus, the size of silica nanospheres was sensitive to the amount of precursor material for the low concentration of TEOS.
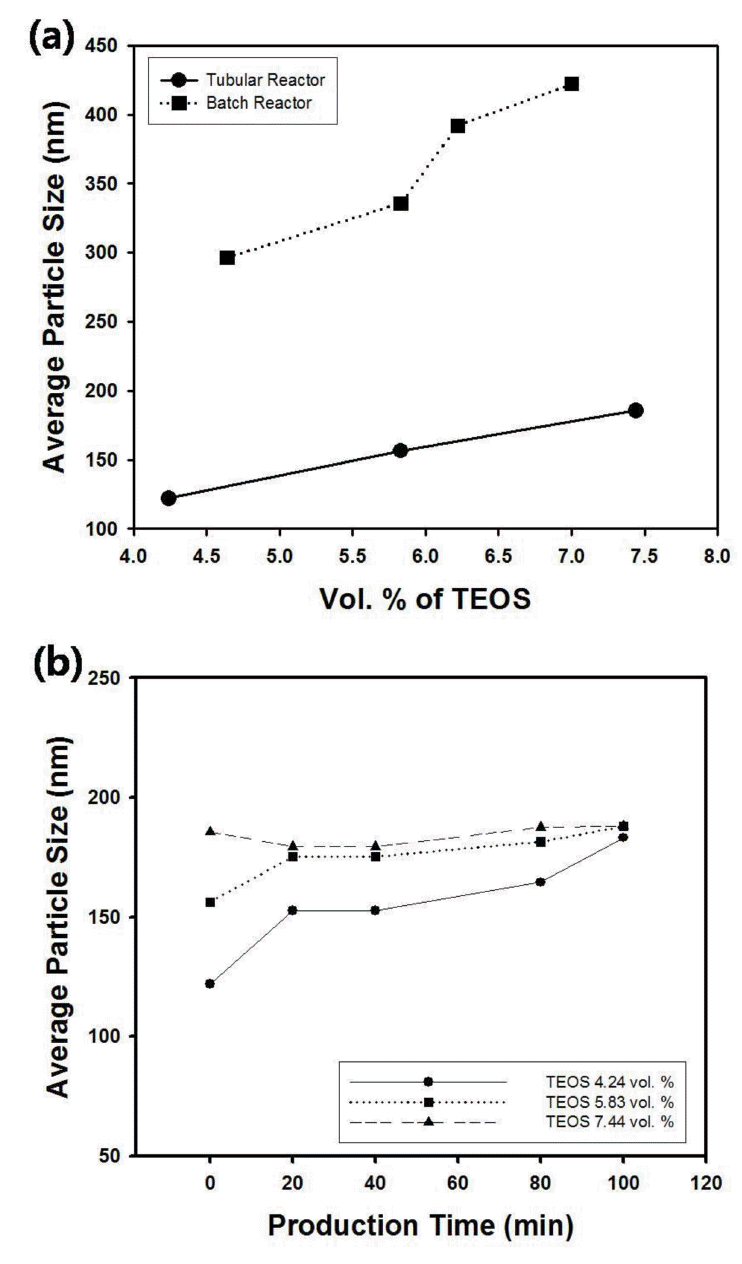
(a) Average particle size of silica nanospheres synthesized using tubular reactor as a function of the amount of TEOS. The amount of TEOS was controlled from 4.24 to 7.44 vol. %. (b) Average particle size of silica nanospheres as a function of TEOS concentration. The dotted line indicates the data from batch-type reactor. For the data from tubular reactor, the production time and injection rates of reactants were fixed as 0 minutes and 100 µl/min, respectively.
In 1994, the tubular type reactors have been adopted for the continuous production of silica particles by Gieshe [14]. His outstanding results are important in that the ratio of seed particles and silica precursor such as TEOS could be adjusted to control the diameter of the silica particles. Unlike the study in this article, Giesche has used silica seeds to grow larger particles with 469 nm in diameter, which is difficult to reach by simple batch type reactors. He could control the size of the silica particles from 135 to 469 nm by adjusting the ratio of silica seed and TEOS in feed streams. Additionally, the concentration of ammonia has been adjusted to control the size of the silica particles from 288 to 422 nm. However, other reaction parameters such as the injection rate, tube diameter, and the length of reactor were changed simultaneously with ammonia concentration. Thus, the research in this article is still important in that the reaction parameters were tuned more systematically to confirm the effect of certain experimental factor with clear trend while fixing the other conditions. Moreover, the effect of mixing conditions of reactants using T-mixer is not studied by previous articles, and it will be described systemically from the following paragraph in this article.
To study the effect of the blade length inside T-mixer on the size distribution of silica particles, two blades were inserted inside T-mixer and continuous production of silica nanospheres were performed using the composition of the reactant feed streams shown in Table 2 with injection rate of 200 µl/min. Figure 8(a) and Figure 8(b) contain the average diameter and polydispersity index (PDI) of the silica particles as a function of production time with or without blades inside T-mixer, respectively. Due to the poor mixing of the reactants without blade inside T-mixer, the average particle size of silica nanospheres was smaller than the diameter of the particles produced using blade, as displayed in Figure 8(a). In the initial stage of the production of silica suspension without blades, the PDI was measured as larger value than 0.2 as shown in the dotted line in Figure 8(b), implying that the size distribution of the particles is broad, when the continuous synthesis was performed without blade inside T-mixer. However, as shown in the solid line of Figure 8(b), relatively small values of PDI were observed when two blades were used in the T-mixer according to the mixer configuration of Figure 2(b). Since the two feed streams of the reaction system can be thoroughly mixed together by passing through the blades inside T-mixer, the particle production with uniform size distribution can be expected and smaller PDI values could be obtained as shown in the graph of Figure 8(a). However, the through mixing of the reactant feed streams could not be expected when the blades were not used in T-mixer, resulting in the broad distribution of the particles. For this case, the two feed streams can be mixed by just diffusion mechanism due to concentration gradients. It is possible to apply nucleation and growth model for the formation of the particles with monosized diameter. For the complete mixing of the reactants, nucleation of the silica particles occurs only in the early stage of the reaction within short time interval, causing the generation of monodisperse silica particles. On the contrary, prolonged nucleation due to incomplete mixing of the reactants and further growth of the particles will make undesired effect to the monodispersity of the colloidal silica, causing polydisperse particles during the synthesis step.
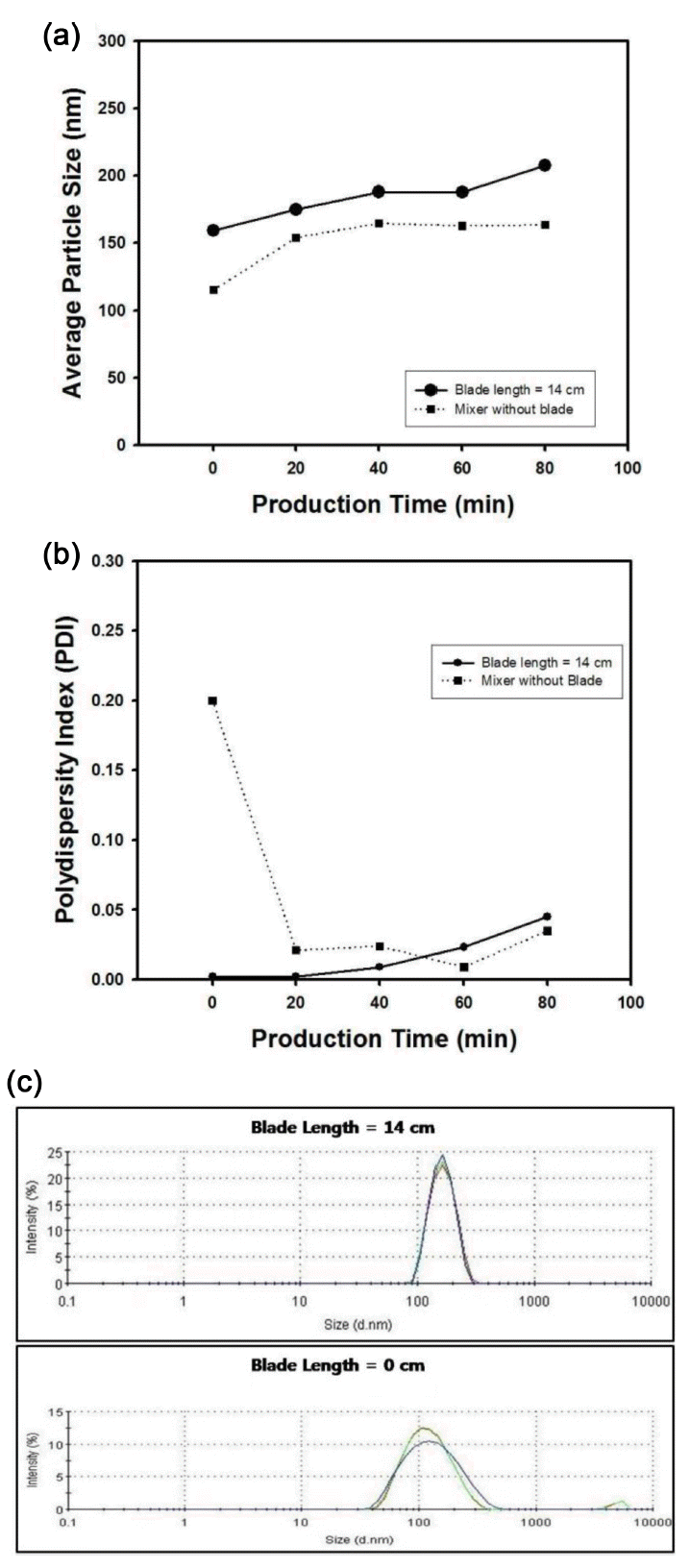
(a) Average particle size and (b) Polydispersity index (PDI) of silica nanospheres as a function of production time. The length of blade in T-mixer was fixed as 0 cm or 14 cm. (c) Particle size distribution of silica nanosphere. The mixer length was fixed as 0 cm or 14 cm. The injection rate of feed streams was fixed as 200 µl/min.
Figure 8(c) contains the size distribution of silica particles synthesized with or without blades in T-mixer for the production time of 0 minutes, just after the start-up of the particle elution out of the tubular reactor. The injection rate of the feed streams and tube length were fixed as 200 µl/min and 7.5 m, respectively, for the reactant composition in Table 2. As shown in the PDI data in Figure 8(b), the size distribution of silica particles is quite monodisperse when two blades were inserted inside T-mixer. However, polydisperse particles were observed for the T-mixer without blades due to the failure of initial mixing.
In this study, the effect of blade length was also studied especially for the size distribution of the silica particles synthesized inside tubular reactor in continuous manner. In chemical industry, blade-type insets have been adopted for the enhancement of mixing of liquid feeds or the heat transfer inside tube [15]. Using T-mixer containing blades, through mixing of liquid feeds near the wall and center of the tube can be expected and uniform reaction conversion can be achieved. Figure 9(a) contains the average diameter of silica particles as a function of production time with one or two units of blades inserted inside T-mixer. The injection rate of feed streams was fixed as 200 µl/min for both experiments. By changing the blade length inside T-mixer, the average size of silica particles was maintained as about 180 nm in diameter, although the production time changed from 0 to 80 minutes. However, the variation of the PDI values of silica particles was drastically fluctuated as the production time was varied from 0 to 80 minutes when one unit of blade was used inside T-mixer, as indicated with dotted line in the graph of Figure 9(b). Thus, it is evident that the mixing length of the reactant streams is crucial factor for the synthesis of the silica particles with uniform diameter by continuous reaction system. This can be clearly confirmed from Figure 9(c), which contains the size distribution of silica particles for the production of 0 minute, which were produced using one or two units of blades inside T-mixer. Thus, under the same synthesis conditions, longer mixing length of T-mixer will cause more monodisperse silica particles the uniform size distribution.
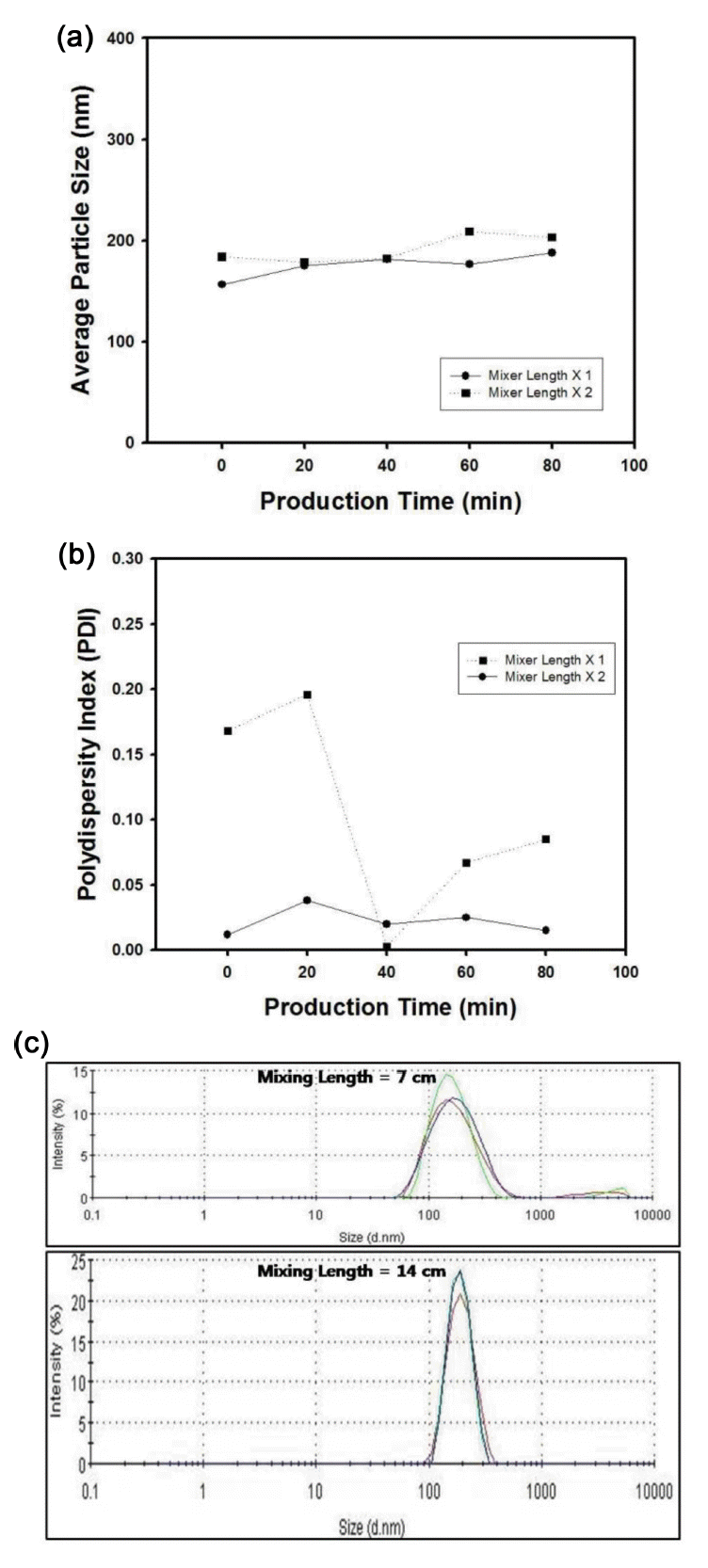
(a) Average particle size of silica nanospheres as a function of production time. The length of blade in T-mixer was fixed as 7 cm or 14 cm. (b) Polydispersity index (PDI) of silica nanospheres as a function of production time. The length of blade in T-mixer length was fixed as 7 cm or 14 cm. (c) Particle size distribution of silica nanospheres. The mixer length was fixed as 7 cm or 14 cm. The injection rate of feed streams was fixed as 100 µl/min.
In this article, the configuration of blades inside T-mixer was also changed to study its effect on the diameter of silica particles as a function of production time. Figure 10(a) contains the schematic figure of ‘configuration 2’ for the T-mixer combined with tubular reactor. In ‘configuration 2’, one blade unit (7 cm in length) was inserted inside T-mixer which is connected with inlet position of tubular reactor and another blade (7 cm in length) was inserted in the middle of the Teflon tube, as described in Figure 10(a). On the contrary, ‘configuration 1’ is composed of two units of blades (14 cm in length) inside T-mixer which is connected with the input part of the tubular reactor, as depicted in Figure 2(b). For these two mixer configurations, the change of particle size of silica suspension was compared together as a function of production time, as displayed in the graph of Figure 10(b). For both configurations, the length of tube and injection rate of the reactant streams was fixed as 7.5 m and 200 µl/min, respectively, for the reactant composition shown in Table 2. For ‘configuration 1’, the reactant streams are mixed mainly in the early stage of retention period inside tubular reactor, since the two blades for mixing are connected in the inlet part of the Teflon tube. However, in ‘configuration 2’, the mixing parts are separated in continuous reaction system, in which the reactant streams are mixed in the input part of the reactor and the mixing is induced once again in the middle of the Teflon tube. Figure 10(b) shows that the size of silica particles was measured as similar values and there are minor differences of particle size between for both configurations as a function of production time. However, the size of silica particles grows continuously until the production time of 80 minutes for the case of ‘configuration 1’, whereas the particle diameter reaches saturated values such as about 150 nm when ‘configuration 2’ was used in the continuous production system. For both cases, the size distribution of the particles was almost monodisperse as contained in the graph of Figure 10(c), since the total mixing length was the same for both configurations. Figure 10(d) contains the scanning electron microscope image of silica nanospheres synthesized using tubular reactor with ‘configuration 2’ when the production was 80 minutes. The size of the particles could be estimates as about 150 nm, which is comparable to the result of particle size analyzer.
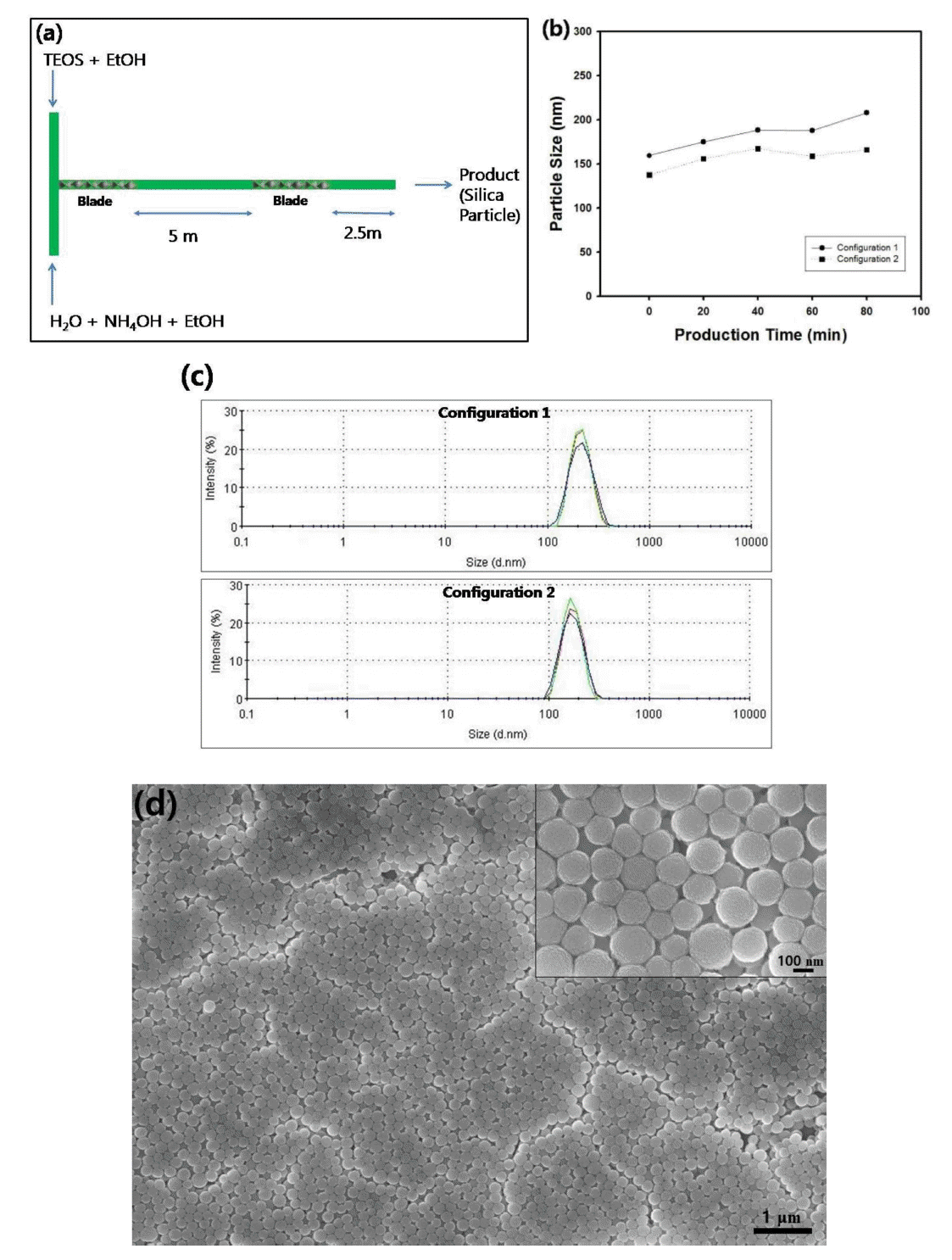
(a) Schematic figure for the configuration of T-mixer with mixing units (blades) attached to the inlet position and middle of the Teflon tube. (b) Average particle size of silica nanospheres as a function of production time. The length of blade in static mixer was fixed as 14 cm and the configuration of mixers were changed as ‘configuration 1’ or ‘configuration 2’. (c) Particle size distribution of silica nanospheres. The length of static mixer was fixed as 14 cm and the mixer configuration was changed as ‘configuration 1’ or ‘configuration 2’. The injection rate of feed streams was fixed as 200 µl/min. (d) Scanning electron microscope image of silica nanospheres synthesized using tubular reactor with ‘configuration 2 when the production time was 80 minutes.
The scale-up of the tubular reactor system is essential for industrial production of silica particles with large quantity. In this case, the diameter of the tubular reactor as well as tube length should be enlarged together, indicating that the mixing of the reactant streams can be a critical issue since the concentration of reactants will change as a function of radial direction. To enhance the production rate of the silica particles, the injection rate should be also increased for the industrial uses. In both cases, T-mixer can be chosen as the mixing device of the reactants, and the optimum configurations should be found for scale-up. In fact, the continuous production can be more plausible solution for the industrial synthesis of silica particles compared to batch reactors since the particles can be produced in longer period of time in continuous fashion. Though the amount of one product stream cannot be enough, multi-tube line can be adopted to produce larger quantity of the particle suspension for practical purpose.
Figure 11(a) contains the average particle size of silica nanospheres as a function of production time with three kinds of solvent medium such as methanol, ethanol, and isopropanol. For the case of continuous synthesis of silica particles using ethanol as dispersion medium, the particle size variation was negligible with the increase of production time, whereas the other solvents induced fluctuation of particle diameter, as displayed in the graphs of Figure 11(a). The particle size increased with the molecular weight of alcohol increased from methanol (CH3OH) to isopropanol (CH3CH2CH2OH), since the stability of larger particles increases as the dielectric constant of the solvent medium increases [16,17]. Moreover, the miscibility of heavy alcohols with water and TEOS is not comparable with that of alcohols having small molecular weight, implying the increase of particle size of the silica nanospheres can be promoted [18]. Thus, the average particle size of silica nanospheres increased when long chain alcohol was used as the reaction medium of the synthesis reaction, indicating that the particle size can be also controlled by changing the reaction medium with different alcohols for Stober reaction.
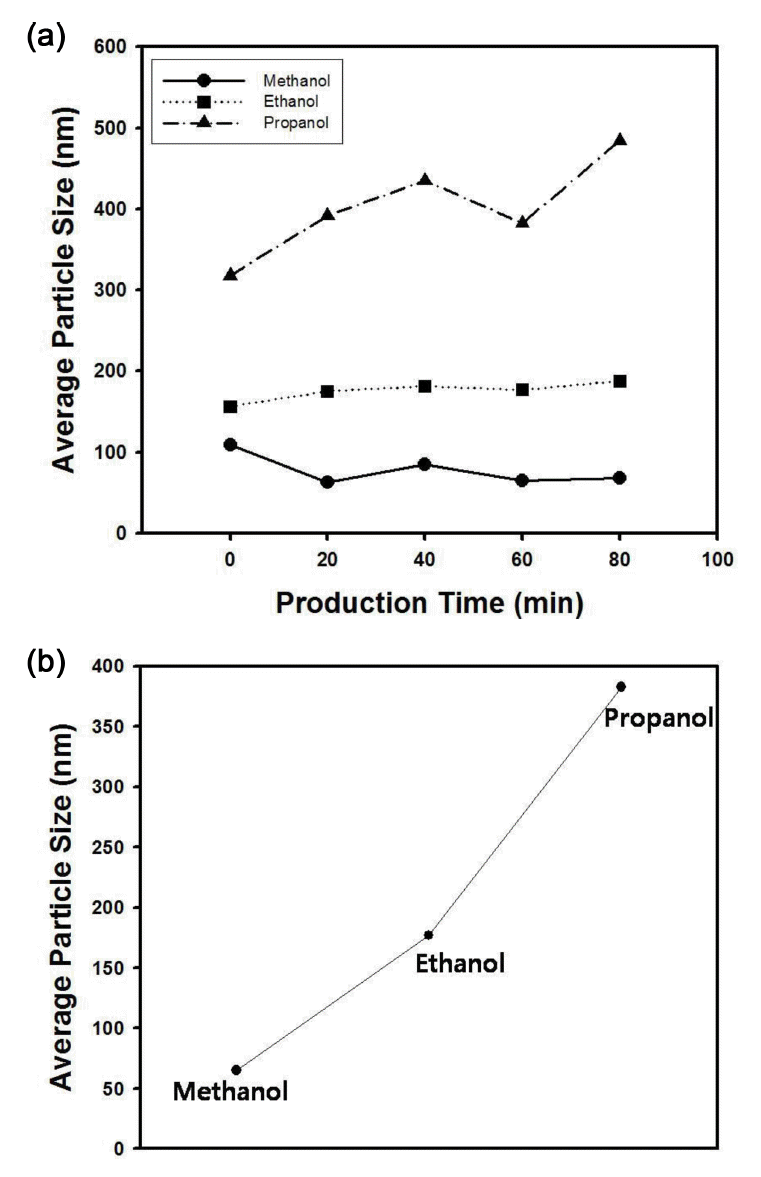
(a) Average particle size of silica nanospheres with different solvent medium. The length of blade in T-mixer was fixed as 7 cm and the injection rate of feed streams was maintained as 100 µl/min. (b) Average particle size of silica nanospheres with different solvent medium when the production time was fixed as 40 minutes.
Though the particle diameter in this study is limited to 500 nm, micron-sized particles can be also synthesized as continuous manner, as reported by recent studies. Recently, monodisperse silica particles larger than 1 μm could be obtained by adjusting reaction compositions or multiple injection of TEOS in tubular reaction system [19,20]. To avoid clogging of the tubular reactor, surfactant can be adopted for enhancement of dispersion stability [19]. Because bare silica particles are coated with silanol groups, it is necessary to functionalize the particles with proper silane coupling agents in continuous reactor. To this end, additional reaction site can be attached to the continuous reaction system for post-functionalization step, as reported recently [21]. Thus, the results in this study can be expanded for wide range of particle size and surface functionalization.
Because unreacted precursor, ammonia catalyst, and water are mixed with silica nanospheres contained in outlet stream, the additional growth of particles is possible after collection of the products in exit stream. Thus, centrifugation of the products to exclude remaining reactants from the silica nanospheres is necessary to avoid unexpected growth of the particles. In addition to centrifugation, adjustment of pH can be considered after the collection of the particle suspension by adding acid to obtain neutral pH and terminate sol-gel reaction. The silica nanospheres synthesized in this study can be applied to separation technologies such as adsorption and oil removal as well as function coating materials like superhydrophobic films [22-24].
4. CONCLUSIONS
Monodisperse silica nanospheres were synthesized using tubular reactor for the continuous production of the particle suspension. The optimum tube length was determined as 7.5 m in which the particle diameter reached saturated value at the injection rate of 85 µl/min. As retention time of the reactant streams increased inside tubular reactor, the particle size also increased due to the sufficient reaction time of the raw materials. The fluctuation of the particle size was observed as a function of production time for short retention time, whereas relatively uniform diameter of the particles could be obtained for the retention time larger than 125 minutes.
The size of silica nanospheres was not sensitive to the variation of the reactant compositions such as ammonia and water for tubular reactor compared to the case of batch-type reactor. Moreover, the controllable size range of the particles was relatively small for the tubular reactor due to the absence of shear-induced flocculation mechanism unlike batch-type reactor with mechanical stirring.
The length of screw-type blade inside T-mixer affected mainly to the size distribution of silica nanospheres, and monodisperse silica particles could be obtained by the mechanism of static mixing of reactant streams inside tubular reactor. The configurations of the blades in tubular reactor and the effect of alcohol chain length in reaction medium were also studied to examine the size distribution and average diameter of the silica particles. Collectively, the size of silica nanospheres could be controlled from 115 to 310 nm using tubular reactor with polydispersity (PDI) range from 0.002 to 0.25.
Acknowledgements
This research was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) (No. NRF-2017R1A6A1A03015562), the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1F1A1047451), Collabo R&D Program supported by Ministry of SMEs and Startups in Korea (S3249683), and Korea Institute for Advancement of Technology (KIAT) grant funded by the Korea Government (MOITIE, P0002007, The Competency Development Program for Industry Specialist).
